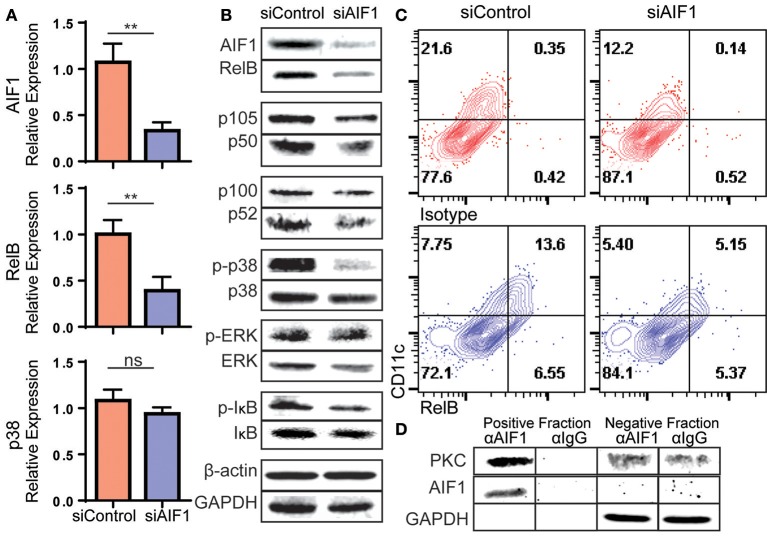
Figure 6.
Loss of AIF1 inhibits RelB expression and p38 signaling during GM-CSF-induced DC differentiation. Murine bone marrow cells were sorted for Lin−CD117+ hematopoietic progenitors. These cells were silenced for AIF1 prior to stimulation with GM-CSF to generate DC. Cells harvested on day 6 were assessed by (A) qPCR for gene expression profiling of AIF1, RelB, and p38 or (B) western blot for protein expression of AIF1, RelB, p105/50, or p100/52. GAPDH and β-actin served as internal loading controls and for normalization. For evaluating phosphorylation of proteins, phosphorylated-IκB (p-IκB), -p38 (p-p38), and -ERK (p-ERK) were measured and normalized to total IκB, p38, and ERK protein, respectively. (C) Flow cytometric dot plots of control or AIF1 siRNA treated cells show RelB vs. CD11c. Gates were established using isotype controls. (D) Protein-protein interaction of AIF1 and PKC from DC were assessed. The precipitated positive fraction was pulled out with αAIF1 and further probed for PKC. IgG antibody served as isotype internal control. Negative fraction is the flow through. All data is representative of four independent experiments. Error bars for all figures indicate standard errors; **< 0.01 and NS, not significant.