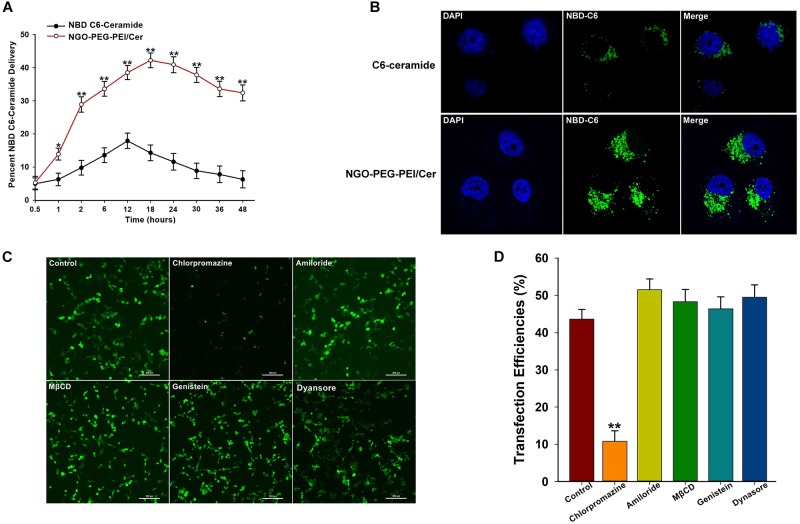
FIGURE 1.
Characterization of NGO-PEG-PEI/Cer complex in vitro. (A) In vitro pharmacokinetics of C6-ceramide delivery by fluorescence spectrophotometry. NGO-PEG-PEI delivery of C6-ceramide resulted in a greater cellular accumulation of C6-ceramide as a function of time relative to naked C6-ceramide administration in the presence of 10% FBS. NGO-PEG-PEI were formulated with trace C6-ceramide to determine the kinetics of ceramide delivery to HCC cells. The total counts of NGO-PEG-PEI and naked C6-ceramide added to the cells were set at 100%. Error bars were based on triplicate samples, ∗p < 0.05, ∗∗p < 0.01 when comparing NGO-PEG-PEI/Cer accumulation with naked C6-ceramide accumulation. (B) Cell confocal microscopic images of NBD C6-ceramide (green) and cell nuclei (blue) were collected from HepG2 cells treated with NGO-PEG-PEI/Cer or C6-Ceramide. (C) To confirm the mechanisms of cellular uptake of the NGO-PEG-PEI/Cer complexes, cells were pre-treated for 30 min with inhibitors diluted in FBS-free media at the indicated concentrations. NGO-PEG-PEI/Cer complexes were then added in the absence or presence of inhibitors for an additional 2 h. Complexes were then removed and replaced with fresh 5% FBS-containing media and incubated for 24 h. Green fluorescence was observed by (C) fluorescence microscopy and (D) flow cytometry. Error bars were based on triplicate samples. ∗∗P < 0.01 versus control.