Abstract
Background
Cardiac rehabilitation improves health-related quality of life (HRQoL) and reduces hospitalizations in patients with heart failure, but international uptake of cardiac rehabilitation for heart failure remains low.
Design and methods
The aim of this multicentre randomized trial was to compare the REACH-HF (Rehabilitation EnAblement in CHronicHeart Failure) intervention, a facilitated self-care and home-based cardiac rehabilitation programme to usual care for adults with heart failure with reduced ejection fraction (HFrEF). The study primary hypothesis was that the addition of the REACH-HF intervention to usual care would improve disease-specific HRQoL (Minnesota Living with Heart Failure questionnaire (MLHFQ)) at 12 months compared with usual care alone.
Results
The study recruited 216 participants, predominantly men (78%), with an average age of 70 years and mean left ventricular ejection fraction of 34%. Overall, 185 (86%) participants provided data for the primary outcome. At 12 months, there was a significant and clinically meaningful between-group difference in the MLHFQ score of –5.7 points (95% confidence interval –10.6 to –0.7) in favour of the REACH-HF intervention group (p = 0.025). With the exception of patient self-care (p < 0.001) there was no significant difference in other secondary outcomes, including clinical events (p > 0.05) at follow-up compared with usual care. The mean cost of the REACH-HF intervention was £418 per participant.
Conclusions
The novel REACH-HF home-based facilitated intervention for HFrEF was clinically superior in disease-specific HRQoL at 12 months and offers an affordable alternative to traditional centre-based programmes to address current low cardiac rehabilitation uptake rates for heart failure.
Keywords: Cardiac rehabilitation, health-related quality of life, heart failure, home-based, randomized controlled trial, self-management
Introduction
With important gains in mortality achieved through pharmacological and device therapy in patients with heart failure with reduced ejection fraction (HFrEF) over the past decade,1 the focus is increasingly shifting towards optimizing health-related quality of life (HRQoL).2 Patients are prepared to trade off longevity for an improvement in HRQoL,3 and a Cochrane meta-analysis of exercise-based cardiac rehabilitation in patients with heart failure reported important improvements in HRQoL and a reduction in rehospitalizations.4 International guidelines consistently recommend group- or centre-based cardiac rehabilitation for patients with HFrEF.5–7 However, less than 10% of people with heart failure in the United States of America and less than 20% in Europe participate in cardiac rehabilitation,8,9 prompting a call to explore newer strategies to improve participation and explore the effectiveness of more accessible alternatives to group- or centre-based cardiac rehabilitation.8
Home-based cardiac rehabilitation programmes can widen access and have been shown to be as effective as group- or hospital-based cardiac rehabilitation after myocardial infarction and coronary revascularization, and with similar costs.10 The high cost of treating people with heart failure is well documented,1 but little evidence (five randomized trials) is available on the clinical and cost-effectiveness of home-based cardiac rehabilitation in heart failure.4 Furthermore, none of the home-based interventions have involved caregivers or have been co-developed with patients, caregivers or clinicians. We therefore developed a novel home-based cardiac rehabilitation intervention derived from health behaviour change theory – the Rehabilitation EnAblement in CHronic Heart Failure (REACH-HF) intervention – for people with heart failure and their caregivers, which is facilitated by a healthcare professional.11 We hypothesized that addition of the REACH-HF intervention to usual care would improve disease-specific HRQoL for patients with HFrEF at 12 months' follow-up compared with usual care alone.
Methods
The REACH-HF trial was conducted and reported in accordance with the Consolidated Standards of Reporting Trials guidelines.12 Our full trial protocol has been published elsewhere.13
Study population and design
The REACH-HF trial was a multicentre, two parallel group, randomized, superiority trial in men and women aged ≥ 18 years with a confirmed diagnosis of HFrEF on echocardiography or angiography (i.e. left ventricular ejection fraction < 45%) within the preceding five years. Participants who had undertaken cardiac rehabilitation within 12 months prior to enrolment were excluded, as were those with a contraindication to exercise testing or exercise training. The published protocol provides a full list of patient inclusion and exclusion criteria.13 Participants were randomized to the REACH-HF intervention plus usual care (REACH-HF group) or usual care alone (control group).
Participants were recruited from primary and secondary care settings in four centres in the United Kingdom (Birmingham, Cornwall, Gwent and York). Participants were randomly allocated in a 1:1 ratio, stratified by investigator site and baseline plasma N-terminal proB-type natriuretic peptide levels ( ≤ 2000 vs. > 2000 pg/ml), using minimization to facilitate balance between the groups. Randomization numbers were computer generated and assigned in strict sequence at the point of randomization. To maintain concealment, the Peninsula Clinical Trials Unit used a password-protected, Web-based randomization system to allocate participants after consent was obtained and baseline assessment data entered. Treatment allocation was open label given the nature of the intervention, but outcome assessors were masked to participants' allocations. We kept a record of instances when outcome assessors were inadvertently unmasked by participants during assessment visits. The trial statistician and all investigators were blinded to the outcome data and group allocation until after prespecified statistical analyses were completed and interpretation of results was agreed. Between January 2015 and February 2016, 216 participants were randomized.
The investigation conforms with the principles outlined in the Declaration of Helsinki and the trial was approved by the North West Lancaster Research Ethics Committee (14/NW/1351). All participants provided written informed consent.
Study intervention
A detailed description of the REACH-HF intervention, its development and its theoretical underpinnings is published elsewhere.11 In the trial, participants in both the intervention and control groups continued with medical management and care of heart failure according to local and national guidelines.7,14 In the UK, patients with HFrEF are usually seen by a community heart failure specialist nurse (soon after hospital discharge or at diagnosis, mainly to optimize drug dosages) and their family doctor, and some are followed up by a cardiologist. Most patients with heart failure do not undertake cardiac rehabilitation.
The REACH-HF intervention is an evidence-informed, patient-centred, theory-based, self-care support programme uniquely co-developed with key stakeholders – patients, caregivers and clinicians. This comprehensive intervention includes four core elements (see Figure S1 in the Supplementary Material online):11,13
REACH-HF manual for patients with a choice of two structured exercise programmes: a chair-based exercise and a progressive walking training programme. Patients were advised to exercise ≥3 times per week, starting from their own personal level and gradually building up over 2–3 months in time/distance/walking pace.
Patient ‘Progress Tracker’ – an interactive booklet designed to facilitate learning from experience to record symptoms, physical activity and other actions related to self-care. Patients recorded: (1) how long/far they plan to walk, (2) whether they have done it, (3) how it felt to identify whether they should be moving up or down in effort next time and (4) their weekly steps per minute (pace).
‘Family and Friends Resource’ – a manual for use by caregivers aimed to increase their understanding of heart failure and caregiver physical and mental well-being.
Facilitation by cardiac nurses or physiotherapists, who attended a three-day training course on the use of person-centred counselling and how to tailor the intervention for the patient and their caregiver.
The intervention was delivered at the patient's home via a mixture of face-to-face and telephone contacts over 12 weeks. The first contact was made by the facilitator and future contacts were agreed by the patient and the facilitator at a mutually convenient time. Patient adherence to the intervention was defined as attendance at the first face-to-face contact with the facilitator and at least two facilitator contacts thereafter – at least one of which must have been face to face.
Usual care
Given that the majority of heart failure patients do not receive cardiac rehabilitation,8,9 usual care in this trial was a no cardiac rehabilitation approach that included medical management according to national and local guidelines, including specialist heart failure nurse care.7 Both REACH-HF and control groups received this usual care.
Outcome measures
The primary outcome was disease-specific HRQoL at 12 months measured using the Minnesota Living with Heart Failure Questionnaire (MLHFQ).15 Secondary outcomes were death, hospitalization, generic quality of life (five-dimension EuroQol (EQ-5D-5L) scale),16 psychological wellbeing (Hospital Anxiety and Depression Scale (HADS)),17 exercise capacity (incremental shuttle walk test)18 and physical activity assessed using a GeneActiv accelerometer.19 Additional secondary measures included the HeartQoL questionnaire20 and Self-Care of Heart Failure Index.21
Outcome data were collected from participants during three clinic visits at baseline and four and 12 months and by postal questionnaire at six months. At the baseline clinic visit, after obtaining written consent, we collected sociodemographic data and information on past medical history from the participants' hospital and primary care records, including key comorbidities, New York Heart Association classification,22 concomitant cardiac drugs and presence of implantable cardiac devices.
Adherence to intervention protocols by the facilitators was ascertained through audio recordings of interviews and a fidelity checklist created as part of the intervention development.11,13 The findings of the intervention fidelity assessment are summarized in Supplementary Table S1.
Serious adverse events were recorded and assessed for their relatedness to the trial processes or the REACH-HF intervention. Adverse events and reactions were regarded as serious if they resulted in death, were life threatening or required hospitalization. All serious adverse events were reported to the ethics and data monitoring committees.
The use of care services, including those provided by healthcare professionals in the community and secondary care, was documented at each follow-up visit by participants completing healthcare resource-use questionnaires and by collection of data on concomitant drug usage. A detailed cost-effectiveness analysis will be presented elsewhere.
Statistical analysis
The sample size was based on an effect size that represented the minimal clinically important difference in our primary outcome measure – that is, five points on the MLHFQ.15 With a type I error of 0.05 and power of 90%, 85 participants per group were required to detect a five-point difference in the MLHFQ score, assuming a standard deviation (SD) of 10.4,23 Assuming an attrition rate of 20% (in accordance with the level of attrition seen in previous trials),10 108 participants were required per group.
All statistical analyses were conducted to a predefined analysis plan agreed in advance with the trial management group, trial steering committee and data management committee. Baseline sociodemographic and health-related variables are reported descriptively by treatment arm. The primary analyses for all participant outcomes were based on a between-group, intention-to-treat basis in participants with complete outcome data at 12 months. Outcomes were analysed using linear regression methods, adjusting for stratification variables and baseline score of the outcome variable, where applicable. Secondary analyses were undertaken on participant outcomes as repeated-measures analysis using all follow-up assessment points (four, six and 12 months). In addition, we did a per-protocol analysis and estimated complier average causal effects analysis of the primary outcome using 12-month follow-up data. We used our definition of adherence to the REACH-HF intervention (see above under ‘Study intervention’) to specify the per-protocol population.
Multiple imputation methods were used as a sensitivity analysis to address the issue of missing outcome data at follow-up. The following predefined subgroups were assessed using interaction terms: the two minimization variables used in randomization (centre and N-terminal proB-type natriuretic peptide) plus time since diagnosis of heart failure and presence of participating caregiver.
Serious adverse events are presented descriptively by treatment arm. All between-group outcome comparisons are presented as mean difference with 95% confidence interval (CI). No correction of p values for multiplicity of testing was undertaken. However, the primary outcome analysis was done before all other analyses, and the p values of all subsequent analyses were interpreted in the context of multiple testing. No interim analyses were performed.
Unit costs were applied to resource use reported at the participant level to estimate the delivery costs associated with the REACH-HF intervention.24 Costs are reported in pounds sterling (£) for 2016. All analyses were performed using Stata version 14.1.
Results
Trial population and interventions
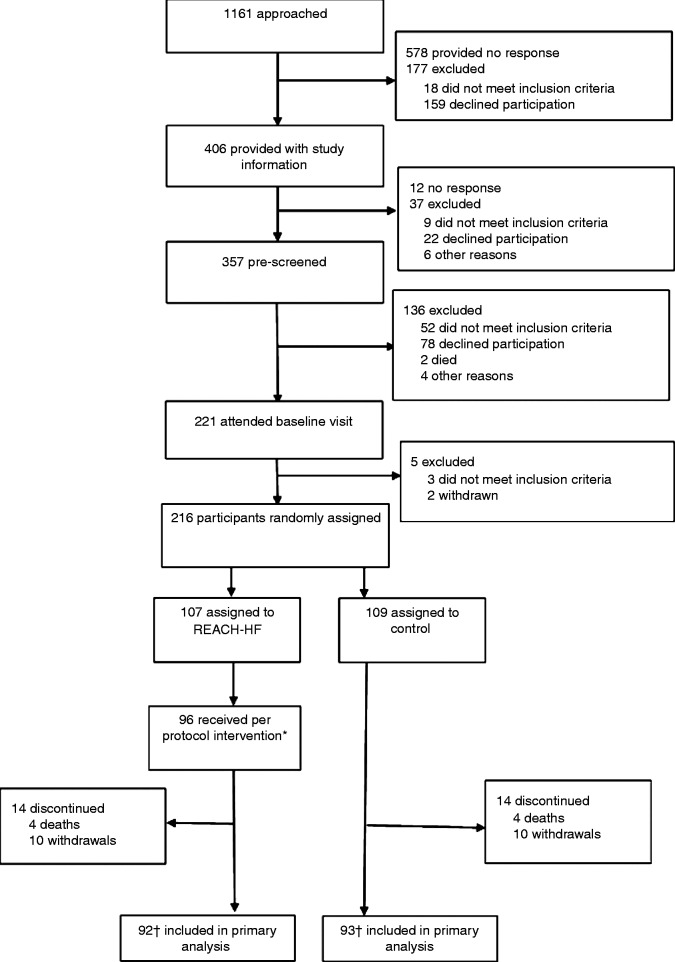
The 216 participants were randomly allocated to the REACH-HF group (n = 107) and control group (n = 109) (Figure 1). Overall, 92 (86%) participants in the REACH-HF group and 93 (85%) in the control group provided data for the primary outcome. Drop out was the result of death (n = 8) or withdrawal (n = 20) – 15 participants did not wish to continue, three were uncontactable and two were too unwell.
Figure 1.
Trial profile.
*Per protocol: REACH-HF participant must attend first face-to-face contact with facilitator and at least two facilitator contacts thereafter, at least one of which must be face-to-face.
†One REACH-HF and two control participants had completed questionnaires insufficiently to allow scoring of primary outcome.
REACH-HF: Rehabilitation EnAblement in CHronic Heart Failure
Participants were predominantly male (78%) and New York Heart Association class II (59%), with an average age of 70 years and mean left ventricular ejection fraction of 34%. Patient-level characteristics at baseline were well balanced between the groups, apart from more frequent cardiac comorbidity (history of myocardial infarction and atrial fibrillation) and, consequently, higher Charlson comorbidity score in the control group (Table 1).25 Mean baseline MLHFQ scores for the REACH-HF group were higher (poorer) than for the control group, but secondary baseline outcomes were similar for the two groups (Tables 2 and 3).
Table 1.
Baseline characteristics. Data are n (%) unless otherwise indicated; percentages may not sum to 100 because of rounding.
| Characteristic | REACH-HF n = 107 | Control n = 109 |
|---|---|---|
| Mean (SD) age, yearsa | 69.7 (10.9) | 69.9 (11) |
| Female sex | 26 (24) | 21 (19) |
| Median (IQR) BMI, kg/m2 b | 28.1 (25.3–32.4) | 28.0 (25–32.2) |
| Main activity | ||
| Retired | 81 (76) | 83 (76) |
| In employment or self-employment | 18 (17) | 17 (16) |
| Living alone | 28 (26) | 22 (20) |
| Ethnic origin | ||
| White | 100 (93) | 104 (95) |
| Other, Black, Asian, other | 7 (7) | 5 (5) |
| NYHA status | ||
| Class I | 24 (22) | 19 (17) |
| Class II | 63 (59) | 63 (58) |
| Class III | 20 (19) | 26 (24) |
| Class IV | – | 1 (1) |
| Ischaemic aetiology of HF | 48 (45) | 50 (46) |
| Time since diagnosis of HF, years | ||
| <1 | 35 (33) | 35 (32) |
| 1–2 | 18 (17) | 20 (18) |
| >2 | 54 (51) | 54 (50) |
| Median (IQR) LVEF, %c | 34.5 (25–39) | 33 (27–36.3) |
| NT-pro-BNP level, pg/ml | ||
| ≤2000 | 84 (79) | 86 (79) |
| >2000 | 23 (22) | 23 (21) |
| Current smoker | 6 (6) | 6 (6) |
| Comorbidities, past or present | ||
| Diabetes mellitus | 26 (24) | 25 (23) |
| Myocardial infarction | 29 (27) | 38 (35) |
| Hypertension | 45 (42) | 42 (39) |
| Chronic renal impairment | 14 (13) | 19 (17) |
| Arthritis, osteoarthritis or rheumatoid | 45 (42) | 35 (32) |
| Atrial fibrillation or atrial flutter | 48 (45) | 60 (55) |
| COPD | 8 (8) | 9 (8) |
| Depression | 27 (25) | 23 (21) |
| Charlson comorbidity score > 3d | 12 (11) | 26 (24) |
| Baseline use of drugs | ||
| Beta-blocker | 90 (84) | 90 (83) |
| Angiotensin II receptor antagoniste | 31 (29) | 24 (22) |
| ACE inhibitore | 68 (64) | 74 (68) |
| Loop diuretic | 70 (65) | 68 (62) |
| Aldosterone antagonist Digoxin | 64 (60) 20 (19) | 52 (48) 14 (13) |
| Baseline use of devices | ||
| ICD | 10 (9) | 11 (10) |
| CRT | 10 (9) | 5 (5) |
| Combined CRT/ICD | 5 (5) | 4 (4) |
| Pacemaker | 11 (10) | 11 (10) |
| Location | ||
| Cornwall, England, UK | 30 (28) | 31 (28) |
| Gwent, Wales, UK | 23 (22) | 23 (21) |
| Birmingham, England, UK | 27 (25) | 28 (26) |
| York, England UK | 27 (25) | 27 (25) |
| Caregiver present at randomization | 53 (50) | 44 (40) |
REACH-HF: Rehabilitation EnAblement in CHronic Heart Failure; SD: standard deviation; IQR: interquartile range; BMI: body mass index; NYHA: New York Heart Association; HF: heart failure; NT-pro-BNP: N-terminal proB-type natriuretic peptide; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease; ICD: implantable cardioverter defibrillator; CRT: cardiac synchronization therapy device; UK: United Kingdom.
National Audit of Cardiac Rehabilitation (NACR) 2013–2014 data for comparison: total mean (SD) age in NACR = 67 (13) years, mean age for patients with heart failure = 69 (13) years.
Numerical values for body mass index available for 215 participants (REACH-HF, n = 107; control, n = 108).
Numerical values for LVEF available for 156 participants (REACH-HF, n = 76; control, n = 80). Categorical data collected for 60 participants. All participants had an ejection fraction <45% or systolic dysfunction.
For the REACH-HF trial, we calculated the Charlson comorbidity score but not the Charlson comorbidity index or Charlson comorbidity adjusted life expectancy, as some of our patient population were older than 80 years, which is the limit for these additional scores.
Patients who were intolerant to angiotensin-converting enzyme inhibitor were on angiotensin II receptor antagonist (e.g. losartan, candesartan).
Table 2.
Primary and secondary patient reported outcomes at baseline and follow-up. Data are mean (standard deviation, n) unless otherwise indicated.
| Outcome | Baseline |
Follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Four months |
Six months |
12 months |
||||||||
| REACH-HF | Control | REACH-HF | Control | REACH-HF | Control | REACH-HF | Control | Between-group difference | p value | |
| MLHFQ | ||||||||||
| Overall | 32.8 (23.8, 107) | 28.3 (22, 109) | 22.7 (18.4, 96) | 27.8 (23.2, 100) | 28.8 (20.5, 90) | 29.5 (21.8, 94) | 24.1 (20.9, 92) | 27.5 (23.2, 93) | –5.7 (–10.6 to –0.7) | 0.025 |
| Physical | 16.5 (11.5, 107) | 14.7 (11.2, 109) | 11.7 (9.0, 96) | 14.5 (11.3, 100) | 14.7 (10.7, 90) | 14.9 (11.2, 94) | 12.2 (10.8, 92) | 14.5 (11.8, 93) | –3.2 (–5.7 to –0.6) | 0.016 |
| Emotional | 7.7 (7.3, 107) | 6.8 (6.6, 109) | 4.8 (5.8, 96) | 6.4 (6.9, 100) | 6.2 (6.2, 90) | 6.8 (6.8, 94) | 5.1 (5.8, 92) | 5.5 (6.4, 93) | –0.8 (–2.2 to 0.6) | 0.273 |
| HADS | ||||||||||
| Anxiety | 5.1 (4.4, 107) | 5.7 (4.3, 109) | 4.4 (3.9, 95) | 5.2 (4.2, 101) | 4.7 (3.7, 89) | 5.4 (4.3, 94) | 4.2 (3.8, 88) | 4.7 (4.5, 92) | 0.1 (–0.8 to 1.0) | 0.829 |
| Depression | 4.4 (3.5, 107) | 4.6 (3.3, 109) | 3.6 (2.7, 95) | 4.5 (3.5, 101) | 4.6 (3.2, 89) | 4.7 (3.6, 94) | 3.6 (3.1, 88) | 3.9 (3.4, 92) | –0.2 (–1.1 to 0.6) | 0.563 |
| HeartQoL | ||||||||||
| Global | 1.8 (0.7, 107) | 1.8 (0.7, 109) | 2.0 (0.7, 95) | 1.9 (0.8, 101) | 1.8 (0.8, 89) | 1.8 (0.8, 91) | 1.9 (0.8, 88) | 1.9 (0.9, 92) | 0.0 (–0.2 to 0.2) | 0.823 |
| Physical | 1.7 (0.8, 107) | 1.7 (0.8, 109) | 1.9 (0.8, 95) | 1.7 (0.9, 101) | 1.6 (0.8, 90) | 1.7 (0.9, 92) | 1.8 (0.9, 88) | 1.7 (0.9, 92) | 0.0 (–0.2 to 0.2) | 0.869 |
| Emotional | 2.1 (0.9, 107) | 2.2 (0.8, 109) | 2.3 (0.8, 95) | 2.2 (0.8, 101) | 2.2 (0.8, 89) | 2.1 (0.8, 93) | 2.3 (0.8, 88) | 2.3 (0.8, 92) | 0.0 (–0.2 to 0.3) | 0.683 |
| EQ-5D-3L | 0.739 (0.234, 106) | 0.723 (0.236, 108) | 0.758 (0.223, 95) | 0.753 (0.219, 101) | 0.708 (0.265, 88) | 0.733 (0.217, 92) | 0.752 (0.240, 88) | 0.739 (0.263, 92) | –0.024 (–0.091 to 0.044) | 0.487 |
| EQ-5D VAS (0 to 100) | 69 (20), 97 | 71 (20), 97 | 73 (17), 90 | 74 (17), 93 | 72 (18), 80 | 70 (19), 85 | 74 (18), 85 | 73 (22), 84 | 1 (–5 to 6) | 0.859 |
| SCHFI | ||||||||||
| Maintenance | 55.8 (16.5, 107) | 54.5 (14.5, 109) | 68.3 (13.6, 96) | 55.7 (17.0, 101) | 65.4 (14.4, 89) | 54.7 (16.0, 94) | 63.8 (17.0, 87) | 55.2 (16.8, 92) | 8.0 (3.6 to 12.4) | <0.001 |
| Management | 43.1 (25.9, 47) | 40.4 (21, 59) | 46.8 (24.2, 33) | 42.0 (21.0, 48) | 52.1 (18.8, 42) | 41.9 (21.6, 37) | 53.8 (23.4, 39) | 43.4 (20.1, 40) | 9.4 (–4.0 to 22.8) | 0.165 |
| Confidence | 61.7 (25.0, 107) | 65.3 (23.8, 108) | 67.0 (22.3, 94) | 64.7 (21.7, 101) | 65.4 (22.8, 85) | 62.5 (22.7, 93) | 70.3 (21.8, 88) | 66.4 (21.3, 92) | 5.6 (–0.1 to 11.3) | 0.056 |
EQ-5D-3L: three level version of five-dimension EuroQol scale; HADS: Hospital Anxiety and Depression Scale; MLHFQ: Minnesota Living with Heart Failure Questionnaire; REACH-HF: Rehabilitation EnAblement in CHronic Heart Failure; SCHFI: Self-Care of Heart Failure Index; VAS: visual analogue scale
Table 3.
Secondary objective outcomes at baseline and follow-up. Data are mean (standard deviation, n) unless otherwise indicated.
| Outcome | Baseline |
Follow-up |
||||||
|---|---|---|---|---|---|---|---|---|
| Four months |
12 months |
|||||||
| REACH-HF | Control | REACH-HF | Control | REACH-HF | Control | Between-group difference | p value | |
| ISWT, m | 262.3 (153.4, 99) | 239.7 (152.4, 103) | 328.5 (181.3, 66) | 294.3 (215.5, 75) | 328.5 (181.3, 66) | 294.3 (215.5, 75) | 0.1 (–33.3 to 33.5) | 0.995 |
| Number of days/week with at least 10 min/day activity >100 milli-ga | 5.8 (2.3, 99) | 5.9 (1.9, 103) | 5.6 (2.4, 78) | 5.5 (2.6, 84) | 5.6 (2.4, 78) | 5.5 (2.6, 84) | 0.2 (–0.4 to 0.7) | 0.601 |
| Average time/day (min) | ||||||||
| ≤20 milli-ga | 1104 (102, 99) | 1106 (114, 103) | 1107 (110, 88) | 1092 (116, 93) | 1092 (124, 78) | 1103 (118, 84) | –7 (–29 to 15) | 0.534 |
| 21–40 milli-ga | 141 (35, 99) | 136 (35, 103) | 140 (35, 88) | 138 (30, 93) | 142 (39, 78) | 138 (34, 84) | –1 (–9 to 8) | 0.880 |
| 41–60 milli-ga | 80 (25, 99) | 80 (27, 103) | 80 (27, 88) | 82 (26, 93) | 81 (30, 78) | 81 (28, 84) | 0 (–6 to 6) | 0.901 |
| 61–80 milli-ga | 45 (21, 99) | 46 (21, 103) | 45 (22, 88) | 48 (22, 93) | 48 (23, 78) | 46 (22, 84) | 2 (–2 to 5) | 0.372 |
| 81–100 milli-ga | 26 (16, 99) | 27 (16, 103) | 26 (16, 88) | 28 (17, 93) | ||||
| >100 milli-ga | 42 (34, 99) | 46 (40, 103) | 43 (37, 88) | 51 (46, 93) | ||||
1000 milli-g = 1 g = 9.81 m/s2, < 40 milli-g is approximately equivalent to sedentary activities such as sitting, lying and ≥100 milli-g is approximately equivalent to activities undertaken at a moderate to vigorous intensity.
REACH-HF: Rehabilitation EnAblement in CHronic Heart Failure; ISWT: incremental shuttle walk test; milli-g: milli-gravity unit
Of the 107 patients randomized to the REACH-HF group, 96 (90%) met our definition of intervention adherence.
Primary outcome: disease-specific HRQoL
At 12 months, MLHFQ total scores improved in the REACH-HF group but did not change in the control group, with a significant between-group difference of –5.7 points (95% CI –10.6 to –0.7) in favour of the REACH-HF group (p = 0.025; Table 2). This difference was also consistent across per-protocol, complier average causal effects, multiple-imputation and repeated-measure analyses. The MLHFQ physical score also differed significantly in favour of the REACH-HF group (mean difference at 12 months –3.2 (95% CI –5.7 to –0.6, p = 0.016)) but the MLHFQ emotional score did not (–0.8 (–2.2 to 0.6), p = 0.273). A post-hoc analysis showed that 48 (52%) participants in the REACH-HF group and 31 (33%) in the control group achieved a reduction of ≥5 MLHFQ points.
Secondary outcomes
The maintenance score on the Self-Care of Heart Failure Index, a measure of self-care, was in favour of the REACH-HF intervention group at 12 months (p < 0.001). Within-group improvements from baseline were seen in the REACH-HF group for HADS anxiety and depression, incremental shuttle walk test and Self-Care of Heart Failure Index (management and confidence) but did not reach statistical significance compared with control at 12 months. No differences were seen in the other secondary outcomes, that is, EQ-5D, HeartQoL and physical activity (Table 2). Similar patterns of primary and secondary results were seen at four and six months. We found no evidence of a significant subgroup treatment interaction on the primary outcome at 12 months by N-terminal proB-type natriuretic peptide level, presence of caregiver, recruitment site or duration of heart failure (see Supplementary Table S2).
Over the 12 months of the trial, eight (4%) participants died: four deaths in each group and four deaths related to heart failure (one REACH-HF, three controls). In the REACH-HF group, 19 participants had at least one hospital admission during follow-up to 12 months compared with 24 patients in the control group (odds ratio (OR) 0.72 (95% CI 0.35 to 1.51), p = 0.386). Three REACH-HF versus six control patients experienced one or more hospital admissions related to heart failure (0.56, 0.13 to 2.33, p = 0.422). Overall, there were 33 admissions (four related to heart failure) in the REACH-HF group and 35 (10 related to heart failure) in the control group. The independent data monitoring committee considered none of the 37 serious adverse events in the REACH-HF to be related to the intervention.
Costs
To calculate costs, facilitator contact sheets were completed at 12 months and were available for 94 (98%) participants in the REACH-HF intervention group. The mean number of facilitator contacts was 6.5 per participant, and total contact time and non-contact time inputs were 5.3 and 2.9 h per participant, respectively, with overall time input at 8.25 h per participant. Taking into account these contact times, facilitator training, and travel and consumables, the mean total cost for delivery of the REACH-HF intervention was estimated at £418.39 per participant (Supplementary Table S3).
Discussion
In this randomized, multicentre trial, participants with HFrEF who received the novel REACH-HF home-based cardiac rehabilitation intervention for 12 weeks in addition to usual care had superiority in disease-specific HRQoL and self-management at 12 months compared with usual care alone. The magnitude of improvement in total MLHFQ (mean between group difference –5.7 (95% CI –10.6 to –0.7) points) was not only statistically significant but also clinically meaningful (i.e. a reduction ≥ 5 points).16 The MLHFQ score is a key outcome indicator for patient well-being that has been shown to be independently related to survival.26 The cost of the REACH-HF intervention (£418.39 per participant) falls within the National Health Service tariff for cardiac rehabilitation in England of £477 per patient.27
The REACH-HF intervention was also associated with better patient ratings of self-care maintenance assessed using the Self-Care of Heart Failure Index, indicating enhanced engagement in activities such as monitoring their weight and increased exercise, looking for signs of fluid retention and using a system to help remember daily drugs.21
The results of our trial are consistent with existing evidence on the impact of cardiac rehabilitation for heart failure. The 2014 Cochrane meta-analysis of exercise-based cardiac rehabilitation included 33 trials and reported a mean benefit in MLHFQ score of –5.8 (95% CI –9.2 to –2.4) points (p = 0.0007) compared with control.4 However, it is important to note that most of this evidence came from trials of hospital- and centre-based models of cardiac rehabilitation, as only six of the included trials (413 participants) assessed cardiac rehabilitation undertaken exclusively in a home-based setting.4 Furthermore, our findings are in keeping with recent studies that support the use of home-based interventions as an alternative to centre-based cardiac rehabilitation.28,29 Mobile and internet modes of delivery may offer the opportunity to improve cardiac rehabilitation uptake in the elderly.30
We believe that this study is the first randomized trial of a home-based cardiac rehabilitation intervention for heart failure derived from health behaviour change theory and that was co-developed with patients, caregivers and clinicians. We recently published the findings of a single centre pilot trial which supported the feasibility and acceptability of the REACH-HF intervention in heart failure patients with preserved ejection fraction (HFpEF) and indicates that it would be possible to recruit and retain participants in a full randomized trial of our intervention in patients with HFpEF.31
This multicentre trial in patients with HFrEF recruited to target, had excellent intervention adherence (90%) and had a relatively low level of attrition ( < 15% loss) over the 12 months of participant follow-up. There are a number of possible explanations for the lack of a significant between-group difference in other outcomes. First, participants may have insufficiently engaged with the REACH-HF intervention to stimulate an improvement in outcomes. For example, failure to adequately engage in the exercise training programme would explain the lack of between-group difference in exercise capacity and physical activity. Second, the trial was not formally powered to detect differences in secondary outcomes, in particular clinical events. Third, REACH-HF is a comprehensive, multifactorial intervention, with individual patients likely to have experienced different pathways to improved HRQoL, which may include reduced stress or anxiety; improved pacing of physical activity, exercise capacity, or sleep quality; and better medication management. In addition, the baseline characteristics of our study population indicated high levels of comorbidity. The lack of impact on exercise capacity and physical activity may therefore be attributed to the ‘heavy burden of comorbid disease’ that can affect outcomes in older patients with heart failure.32 For example, substantive numbers of patients had atrial fibrillation/flutter (50%) and osteoarthritis or rheumatoid arthritis (37%), which could have limited the participants’ intensity and frequency of exercise and physical activity. Finally, there is a growing evidence base demonstrating the limited sensitivity of the EQ-5D in mild-to-moderate heart failure.33–35 Consistent with our study, the HF-ACTION study found no difference in the EQ-5D utility score after exercise-based cardiac rehabilitation compared with control at 12 months.35 In contrast, the EQ-5D has been shown to be a valid and sensitive measure in patients with advanced heart failure.2
Study limitations
This study had potential limitations. First is the lack of blinding – given the nature of the intervention and control, we could not mask participants to treatments, so our results may reflect patient expectation bias. However, we used self-reported outcome measures and outcome assessor blinding procedures to reduce researcher assessment bias. Second, around 15% of data were missing for the primary outcome measure at follow-up. However, our sensitivity analyses show that the between-group inferences in our trial were robust to data imputation. To take account of the observed baseline between-group imbalance, we adjusted all analyses for baseline outcome scores and the presence of cardiac morbidity (myocardial infarction, atrial fibrillation and atrial flutter). Third, the assessment of adherence is notoriously challenging in home-based interventions (given the self-direct nature of the intervention, we were not a able to capture consistent patient-level data on their level of intervention adherence, such as their exercise training programme).36
Conclusions
The REACH-HF home-based cardiac rehabilitation intervention for the management of HFrEF results in superior and clinically important improvements in disease-specific HRQoL and self-management. These findings support the benefits of an affordable, novel home-based cardiac rehabilitation intervention that offers patients, clinicians and healthcare commissioners an additional option to centre-based cardiac rehabilitation to address current low rates of uptake.
Supplemental Material
Supplemental material for The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial by Hasnain M Dalal, Rod S Taylor, Kate Jolly, Russell C Davis, Patrick Doherty, Jackie Miles, Robin van Lingen, Fiona C Warren, Colin Green, Jennifer Wingham, Colin Greaves, Susannah Sadler, Melvyn Hillsdon, Charles Abraham, Nicky Britten, Julia Frost, Sally Singh, Christopher Hayward, Victoria Eyre, Kevin Paul, Chim C Lang and Karen Smith in European Journal of Preventive Cardiology
Acknowledgements
We thank all participants, facilitators, clinicians, researchers and administrators in Birmingham, Cornwall, Gwent, HM Department NHS Lothian (Carolyn Deighan and Jenny Elliott), Peninsula Clinical Trials Unit, Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), the Programme/Steering Committee (Martin Cowie (chair), Graham Dunn, Suzanna Hardman, Roger Boyle and Liz Clark), the Data Monitoring Committee (Ann Dorthe-Zwisler (chair), Alan Montgomery and Gill Furze) and independent adjudicators (Iain Squire, Sern Lim and Paco Leyva). We thank Jemma Lough for technical editing and John Cleland, John Campbell, Tony Mourant for comments on earlier drafts of this manuscript. Previous presentations: ESC Heart Failure Annual Congress – Vienna, Austria – May 2018 (poster); British Cardiovascular Society annual conference – Manchester, UK – June 2018 (poster and oral). Data sharing: All of the individual participant data collected during the trial (including the data dictionary) will be available, after deidentification, immediately after completion of trial funding (end of June 2018) with no end date. The trial protocol has been published. All proposals requesting data access will need to specify how it is planned to use the data, and all proposals will need approval of the trial's co-chief investigators (HMD and RST) before data release.
ISRCTN86234930
Author contribution
HMD and RST contributed equally. HMD, RST, KJ, RCD, PD, JM, RvL, CGreen, JW, NB, CGreaves, CA and SSingh designed the trial, were responsible for its conduct and obtained the trial funding. CGreaves led the design of the intervention, with strong contributions from JW, PD, the Heart Manual Department (Edinburgh) and the REACH-HF service user advisory group. VE and CH were responsible for trial and data collection management. FW, SSadler, CGreen and MH analysed the data. KP provided patient and public involvement. All authors including, CCL, KS and SSadler, contributed to writing and editing the manuscript, with the lead taken by HMD and RST. All authors commented on the manuscript and agreed the final version. HMD and RST are the guarantors. The corresponding author had full access to all of the data in the trial and had final responsibility for the decision to submit.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: all authors report grants from the UK National Institute for Health Research (NIHR) during the course of the trial. There are no other declared potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the United Kingdom's National Institute for Health Research (NIHR) Programme Grants for Applied Research (grant number RP-PG-1210-12004). RST and NB are part-funded by the National Institute for Health Research (NIHR) Collaboration for Peninsula Leadership in Applied Health Research and Care. KJ is part-funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West Midlands. SSingh is supported by NIHR CLARCH East Midlands. The funders' peer-review process informed the trial protocol. The sponsor of the trial had no role in trial design, data collection, data analysis, data interpretation, or writing of the report. The views expressed in this publication are those of the authors and not necessarily of the NIHR or United Kingdom's Department of Health and Social Care. All authors report grants from the National Institute for Health Research (NIHR) during the course of the trial.
References
- 1.Braunwald E. The war against heart failure: The Lancet lecture. Lancet 2015; 385: 812–824. [DOI] [PubMed] [Google Scholar]
- 2.Calvert MJ, Freemantle N, Cleland JGF. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail 2005; 7: 243–251. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EF, Johnson PA, Johnson W, et al. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001; 20: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014, pp. CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Chronic heart failure: Management of chronic heart failure in adults in primary and secondary care. Clinical guideline CG108, London: NICE, 2010. [Google Scholar]
- 8.Golwala H, Pandey A, Ju C, et al. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: Findings from Get With The Guidelines–Heart Failure Registry. J Am Coll Cardiol 2015; 66: 917–926. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnason-Wehrens B, McGee H, Zwisler AD, et al. Cardiac rehabilitation in Europe: Results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 2010; 17: 410–418. [DOI] [PubMed] [Google Scholar]
- 10.Dalal HM, Zawada A, Jolly K, et al. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ 2010; 340: b5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greaves CJ, Wingham J, Deighan C, et al. Optimising self-care support for people with heart failure and their caregivers: Development of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) intervention using intervention mapping. Pilot Feasibility Stud 2016; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: Extension to cluster randomised trials. BMJ 2012; 345: e5661. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RS, Hayward C, Eyre V. The clinical effectiveness and cost-effectiveness of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) facilitated self-care rehabilitation intervention in heart failure patients and caregivers: Rationale and protocol for a multicentre randomised controlled trial. BMJ Open 2015; 5: e009994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piepoli MF, Conraads V, Corra U, et al. Exercise training in heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011; 13: 347–357. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society. Minnesota Living with Heart Failure Questionnaire, New York: American Thoracic Society, 2004. . http://qol.thoracic.org/sections/instruments/ko/pages/mlwhfq.html (accessed Jan 31, 2018). [Google Scholar]
- 16.EuroQoL G. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 18.Pulz C, Diniz RV, Alves AN, et al. Incremental shuttle and six-minute walking tests in the assessment of functional capacity in chronic heart failure. Can J Cardiol 2008; 24: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Berg-Emons HJ, Bussmann JB, Balk AH, et al. Validity of ambulatory accelerometry to quantify physical activity in heart failure. Scand J Rehabil Med 2000; 32: 187–192. [DOI] [PubMed] [Google Scholar]
- 20.Oldridge N, Hofer S, McGee H, et al. The HeartQoL: Part II. Validation of a new core health-related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol 2014; 21: 98–106. [DOI] [PubMed] [Google Scholar]
- 21.Riegel B, Lee CS, Dickson VV, et al. An update on the self-care of heart failure index. J Cardiovasc Nurs 2009; 24: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, 9th ed Boston: Little, Brown & Co, 1994. [Google Scholar]
- 23.Harrison MB, Browne GB, Roberts J, et al. Quality of life of individuals with heart failure: A randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care 2002; 40: 271–282. . [DOI] [PubMed] [Google Scholar]
- 24.Curtis L, Burns A. Unit costs of health & social care 2016, Canterbury: Personal Social Services Research Unit, 2016. [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 26.Hoekstra T, Jaarsma T, van Veldhuisen DJ, et al. Quality of life and survival in patients with heart failure. Eur J Heart Fail 2013; 15: 94–102. [DOI] [PubMed] [Google Scholar]
- 27.Strategic Commissioning Development Unit. Cardiac rehabilitation: Costing tool guidance, London: Department of Health, 2010. [Google Scholar]
- 28.Kraal JJ, van den Akker-van Marle ME, Abu-Hanna A, et al. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol 2017; 24: 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claes J, Buys R, Budts W, et al. Longer-term effects of home-based exercise interventions on exercise capacity and physical activity in coronary artery disease patients: A systematic review and meta-analysis. Eur J Prev Cardiol 2017; 24: 244–256. [DOI] [PubMed] [Google Scholar]
- 30.Prescott E, Meindersma EP, van der Velde AE, et al. A EUropean study on effectiveness and sustainability of current Cardiac Rehabilitation programmes in the Elderly: Design of the EU-CaRE randomised controlled trial. Eur J Prev Cardiol 2016; 23(2 Suppl): 27–40. [DOI] [PubMed] [Google Scholar]
- 31.Lang CC, Smith K, Wingham J, et al. REACH-HF investigators A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: The REACH-HFpEF Pilot Study. BMJ Open 2018; 8: e019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witham MD, Fulton RL, Greig CA, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: A randomized controlled trial. Circ Heart Fail 2012; 5: 209–216. [DOI] [PubMed] [Google Scholar]
- 33.Kularatna S, Byrnes J, Chan YK, et al. Comparison of contemporaneous responses for EQ-5D-3L and Minnesota Living with Heart Failure; a case for disease specific multiattribute utility instrument in cardiovascular conditions. Int J Cardiol 2017; 227: 172–176. [DOI] [PubMed] [Google Scholar]
- 34.Kularatna S, Byrnes J, Chan YK, et al. Comparison of the EQ-5D-3L and the SF-6D (SF-12) contemporaneous utility scores in patients with cardiovascular disease. Qual Life Res 2017; 26: 3399–3408. [DOI] [PubMed] [Google Scholar]
- 35.Ambrosy AP, Cerbin LP, DeVore AD, et al. Aerobic exercise training and general health status in ambulatory heart failure patients with a reduced ejection fraction—findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial. Am Heart J 2017; 186: 130–138. [DOI] [PubMed] [Google Scholar]
- 36.Bollen JC, Dean SG, Siegert RJ, et al. A systematic review of measures of self-reported adherence to unsupervised home-based rehabilitation exercise programmes, and their psychometric properties. BMJ Open 2014; 4: e005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial by Hasnain M Dalal, Rod S Taylor, Kate Jolly, Russell C Davis, Patrick Doherty, Jackie Miles, Robin van Lingen, Fiona C Warren, Colin Green, Jennifer Wingham, Colin Greaves, Susannah Sadler, Melvyn Hillsdon, Charles Abraham, Nicky Britten, Julia Frost, Sally Singh, Christopher Hayward, Victoria Eyre, Kevin Paul, Chim C Lang and Karen Smith in European Journal of Preventive Cardiology