Figure 2. Hepatic fatty acid transport and metabolism.
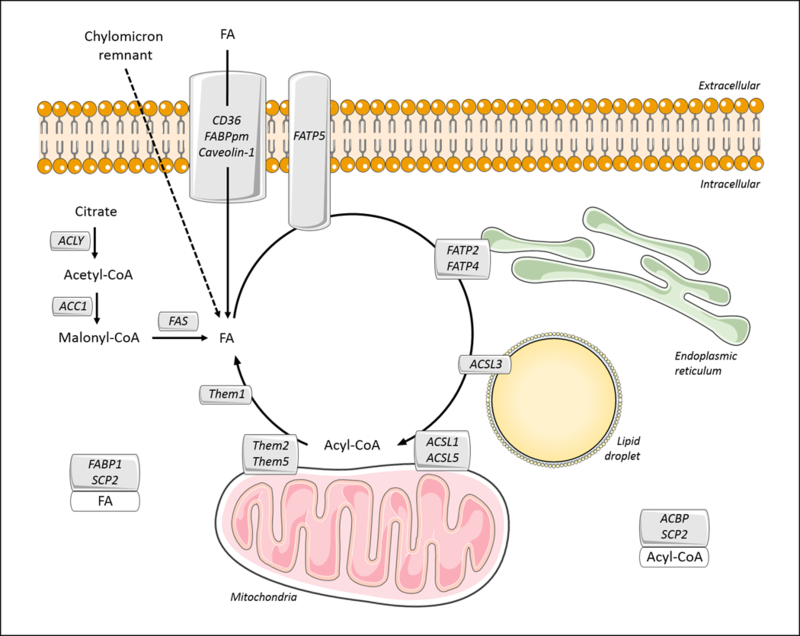
Within the plasma membrane, FA translocase (FAT)/CD36, plasma membrane FA-binding protein (FABPpm), and Caveolin-1 mediate the uptake of fatty acid (FA) that is bound to circulating albumin. Alternatively, hepatic FA can be obtained by the internalization of chylomicron remnant or by de novo lipogenesis. The latter occurs through the activity of three key enzymes: ATP-citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), and fatty acid synthase (FAS). In the cytosol, FAs are bound to the fatty acid-binding protein-1 (FABP1) and sterol carrier protein-2 (SCP2), which may control their cellular distribution. FA transport proteins (FATP2, 4 and 5) and long-chain acyl-CoA synthetases (ACSL1, 3 and 5) mediate the activation of long-chain FA to acyl-CoA molecules and their channeling to metabolic pathways. Although associated with mitochondria, ACSL5 may function to promote triglyceride biosynthesis. In the cytosol, acyl-CoAs are bound to acyl-CoA-binding protein (ACBP) or SCP2. Acyl-CoA thioesterases (ACOT)/thioesterase superfamily members (Them1, 2 and 5) appear to counteract ACSL activity by catalyzing the hydrolysis of acyl-CoA molecules into FA and CoA. This may provide additional means of controlling the balance between FA and acyl-CoA within hepatocytes.
