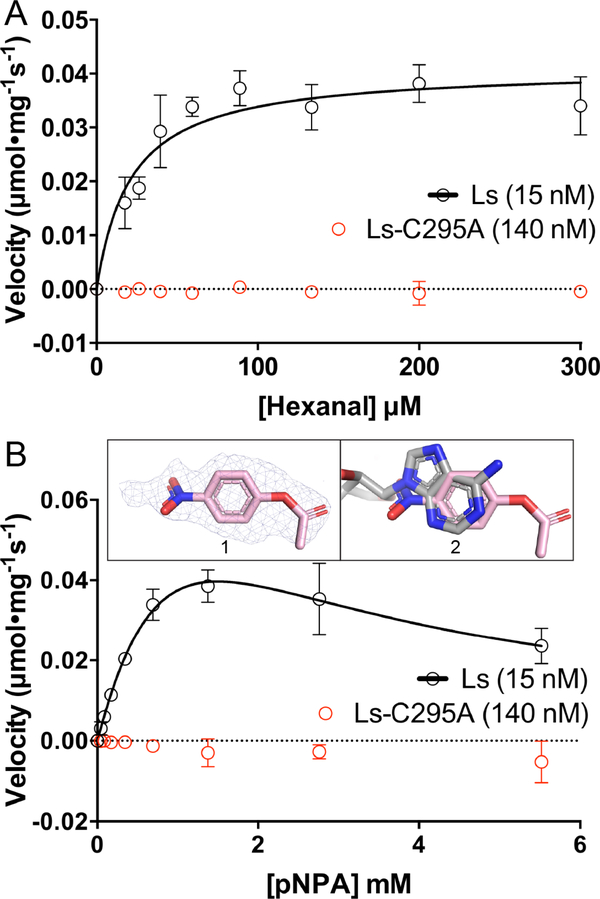
Fig. 7.
Catalytic activity data showing that LsALDH16 is a bona fide enzyme. (A) ALDH activity of LsALDH16 using hexanal as the substrate in the presence of 2.5 mM NAD+ (black). Data for the C295A variant of LsALDH16 are shown in red. (B) Esterase activity of LsALDH16 using pNPA as the substrate in the absence of NAD+ (black). Data for the C295A variant of LsALDH16 are shown in red. Inset 1 shows polder omit electron density (3σ) for pNPA bound to C295A. Inset 2 shows that pNPA occupies the NAD+ adenine site (pNPA in pink, NAD+ in gray). In the activity plots in panels A and B, the data are shown as the mean ± standard error from triplicate measurements. The kinetic parameters are listed in Table 3.