Abstract
Extracellular vesicles (EVs) are membrane vesicles that are released from cells and mediate cell-cell communication. EVs carry protein, lipid, and nucleic acid cargoes that interact with recipient cells to alter their phenotypes. Evidence is accumulating that tumor-derived EVs can play important roles in all steps of cancer progression. Here, we review recent studies reporting critical roles for EVs in 4 major areas of cancer progression: promotion of cancer invasiveness and motility, enhancement of angiogenesis and vessel permeability, conditioning premetastatic niches, and immune suppression.
Introduction
Cells modify their environment and communicate with other cells by many mechanisms, including direct cell-cell contact and secretion of soluble proteins. Recently, release of extracellular vesicles (EVs) from cells has been identified as a major way that cells communicate. While originally thought to be released only from specialized cells (1–3), the past decade has shown that EVs are released not only from all cell types in the human body, but also from virtually all organisms, including bacteria, and parasites (4–10). EVs are referred to by a variety of names including exosomes, microvesicles (MVs), ectosomes, microparticles, and large oncosomes. Generally, the term exosome refers to EVs that are small membrane vesicles (30–150 nm in diameter), formed by vesiculation of intracellular endosomal multivesicular bodies (MVBs) and released by exocytosis (11). MVs, ectosomes, microparticles and large oncosomes are all terms that refer to EVs that bud and are released from the plasma membrane. While exosomes are the most commonly studied type of EV to date (12, 13), increasing recognition of the diversity of EVs is expanding the scope of the field and leading to identification of new functional roles for various types of EVs. For the purposes of this review, we will generally refer to EVs that are shed from the plasma membrane as MVs, noting that these EVs can be of various sizes, including in the same size range as exosomes (30–150 nm) (14–16), intermediate size (150 nm-1 μm) MVs (7, 17, 18), and large oncosomes (>1 μm) (19–21).
EVs have surface molecules that allow them to interact with target cells (22–24). After binding to cells, EVs may modify the physiological state of the recipient cell by directly inducing signaling or alternatively by delivering their internal contents via endocytosis, phagocytosis or fusion with the target cell’s plasma membrane (22, 25–27). EVs carry a variety of bioactive molecular cargoes, such as nucleic acids (DNA, mRNA, microRNA [miRNA], and other non-coding RNAs), proteins (receptors, transcription factors, enzymes, extracellular matrix (ECM) proteins), and lipids that can affect the function and phenotype of recipient cells in diverse ways (7, 25, 28–33).
Recent studies conducting proteomics, lipidomics, and RNA-seq analyses have identified differences in the composition of these bioactive molecular cargoes among diverse types of EVs (34–37). For example, small EVs (typical “exosome” preparation purified at 100,000xg) were found to be enriched in heparin-binding proteins and receptors, including integrins, compared to larger EVs (typical MV preparation purified by centrifugation at 10,000xg), suggesting that they might interact with different target cell populations (34). Likewise, recent studies have found different RNA populations in EVs, with full-length mRNAs >1 kb in length preferentially found in the large EV preparations compared with small EV preparations (36, 38). Although much more characterization of the differences between EV types should be performed, these data suggest that in many cases, the type of EVs may determine which EVs interact with distinct target cells and the functional consequence of those interactions.
One area of intense research is cancer EVs, due to early identification of their role in the tumor microenvironment. In this review, we discuss EV-mediated functions in cancer progression including the classic steps of tumor metastasis and roles in tumor immunity.
Function of EVs in cancer metastasis
Facilitation of tumor cell motility and invasiveness
A first and critical step in cancer metastasis is acquisition of an invasive migratory phenotype, which enables cancer cells to invade surrounding tissue and intravasate into blood and lymphatic vessels. This phenotype involves structural changes of the cancer cell, especially reorganization of the cytoskeleton to form dynamic actin-based invasive structures such as lamellipodia, invadopodia, and amoeboid blebs (39, 40). In addition, changes in the surrounding environment such as altering the phenotype of surrounding non-tumor cells can also greatly contribute to promotion of invasion.
EVs have been shown to carry molecules that enhance migration, and invasion, including matrix metalloproteinases (MMPs), ECM molecules, and growth factors (41–57). Our group found that exosome secretion takes place at matrix-degrading actin protrusions called invadopodia (47) and is critical for invadopodia function, including invadopodia formation, stabilization, and ability to degrade ECM. Notably, we found that exosomes purified from head and neck squamous cell carcinoma (HNSCC) cells carry key invadopodial proteinases, including MT1-MMP and MMP2 (47). Consistent with this finding, secretion of MT1-MMP at invadopodia was found to require the endolysosomal SNARE VAMP7 (58). However, MT1-MMP on exosomes is not critical for the ability of those exosomes to drive invasion through Matrigel (48). In a separate study, we found that autocrine secretion of exosomes also drives cellular motility, in part by carrying fibronectin, which enhances adhesion formation and speed of cancer cells (46). Additional studies have shown that exosomes enhance cancer cell adhesion, motility and invasion (44, 49–51, 59), suggesting that these are major functions of exosomes. In addition, directional sensing of migrating cells has been shown to be dependent on exosome secretion in both cancer cells and neutrophils, suggesting a universal mechanism (46, 54, 60).
MVs have also been shown to carry MMPs and ECM molecules, indicating a diversity of mechanisms by which invasion-promoting molecules can be sorted to EVs (42, 43, 53, 55, 56). For MT1-MMP, delivery into MVs involved association of VAMP3 with the tetraspanin CD9 (43). In some cases, these MVs were shown to directly promote anchorage independent growth (an ECM-sensitive phenotype) and invasion through Matrigel and gelatin (53, 55, 56). In contrast to exosomes, MVs have been shown to be associated with amoeboid motility, downstream of RhoA activation (61). Expression of the constitutively-active G14V mutant of RhoA leads to increased MV blebbing and shedding and induces amoeboid morphology (61), which relates to tumor invasiveness (39, 62). Secretion of tumor-derived large oncosomes also may relate to amoeboid movement (63–65) in that siRNA targeting or chromosomal deletion of Diaphanous-Related Formin 3 (DRH3) affects nonapoptotic blebbing and the release of large oncosomes by changing cortical actin (20) and also affects amoeboid motility.
In addition to an autocrine role for EVs in driving cancer cell migration and invasion, many studies have identified a role for paracrine interactions with host cells in this process. Cancer-derived exosomes are known to convert fibroblasts to an activated myofibroblastic “cancer-associated fibroblast” (CAF) phenotype (66–69). This could increase cancer cell motility by a variety of mechanisms, including synthesis and reorganization of collagen fibers to promote migration (70–75). In addition, fibroblast-secreted exosomes were shown to promote breast cancer cell (BCC) protrusive activity and motility via induction of Wnt-planar cell polarity (PCP) signaling (49). CAF exosomes carrying ADAM10 can also promote cell motility by activating Notch and RhoA signaling in cancer cells (76). Mesenchymal stem cell (MSC)-derived exosomes were reported to promote migration and invasion of gastric cancer cells (77), by activating Akt signaling to induce epithelial-mesenchymal transition and transwell invasion. Finally, CAFs have been reported to deliver miRNAs via EVs that induce aggressive cancer cell phenotypes such as invasiveness, motility, EMT, anchorage-independent cell growth, or drug resistance that could lead to future metastasis (78, 79).
Besides fibroblasts, additional cell types release EVs that facilitate cancer cell invasiveness. This may occur in an ongoing “conversation” via EVs. For example, miR25–3p and miR-92a-3p carried in liposarcoma-derived exosomes were shown to stimulate secretion of IL-6 from tumor-associated macrophages - through miRNA-mediated activation of NF-kB signaling - which in turn promoted liposarcoma cell invasion (80). In ovarian cancer, both chemical inhibition experiments and studies with purified exosomes indicate that exosomal transfer of CD44 to human peritoneal mesothelial cells facilitates cancer invasion. Upregulation of CD44 in the mesothelial cells promoted secretion of MMP9 to directly disrupt the mesothelial barrier (81). Notably, transfer experiments using exosomes purified from control or CD44-knockdown (KD) ovarian cancer cells demonstrated the dependence of the mesothelial cells on exosomal transfer of CD44 for the upregulation of pro-MMP9 secretion (81). Whether the mesothelial MMP9 was actually carried on EVs, as has been shown in other systems (48, 55, 82) was not addressed.
Adipocyte-derived exosomes have been shown to promote migration and invasion of cancer cells (83, 84). In one study, a mechanism was identified and related to alteration of metabolic pathways in the recipient cancer cells (83). Proteomic analysis of the adipocyte exosomes identified multiple proteins associated with fatty acid oxidation (FAO); in the presence of these exosomes, FAO was increased in the melanoma cells. Inhibition of this metabolic pathway completely abrogated the exosome-mediated increase in migration. In obese mice, both the number of exosomes secreted by adipocytes as well as their effect on FAO-dependent cell migration were amplified compared to lean mice. Also in humans, the number of adipose tissue-derived exosomes was positively correlated with body mass index and exosomes from obese individuals increased melanoma migration compared to exosomes from lean individuals.
Platelet-derived EVs (PDEVs), also known as microparticles, have been shown to promote lung cancer cell invasiveness (85), suggesting a role for chronic inflammation and potentially feedback from procoagulant properties of cancer cells. PDEVs transferred integrin CD41/aIIb and stimulated the phosphorylation of MAPK p42/44 as well as the expression of MMP2, MMP9 and MT1-MMP in lung cancer cell lines. In addition to promoting trans-Matrigel chemoinvasion, the PDEVs also increased in vivo metastasis, potentially by “coating” the cells and enhancing invasion at distant sites. Additional studies have shown that PDEVs enhance in vitro invasive behavior and correlate with poor patient outcome ((86) and additional studies reviewed in (87)).
Several remaining issues exist for the function of EVs in tumor cell motility and invasiveness. EVs clearly play an important autocrine role in cell motility and invasion, but the mechanisms are not fully understood. For example, exosomes are known to play a role in cancer cell chemotaxis, but unlike in neutrophils, the responsible exosome cargoes are unknown (46, 54, 60). In addition, the role of EV-associated versus plasma membrane or soluble proteinases in mediating matrix degradation is unclear. For paracrine interactions, the diversity of stromal cells in different organs is high. Thus, it seems likely that there will be a concomitant diversity in EV cargo content and in regulation of tumor cell motility and invasiveness. In addition, in some organs - such as gastrointestinal or skin tissue - the types of stromal cells encountered depend on the organ’s layer structure. A challenge for the future is to understand the role of EV-mediated tumor-host crosstalk in the context of different tissue content and structure.
Role of EVs in promoting angiogenesis and permeability of endothelial cells
Intravasation of tumor cells is a critical step in cancer metastasis that can be enhanced by EVs. A number of studies have shown that EVs enhance both angiogenesis and vascular permeability, which could explain the known “leakiness” of tumor vasculature and enhance cancer intravasation (88–91).
Brain tumors are notoriously vascular and a number of studies have shown that glioma-derived EVs can increase angiogenesis. EVs secreted from glioblastoma cells carry angiogenesis-related proteins such as IL-6, IL-8, angiogenin and IGFBP1, especially under hypoxic conditions, and promote in vitro and in vivo angiogenesis (92, 93). GBM MVs carrying EGFRvIII also induce VEGF expression in endothelial cells, dependent on the presence of EGFRvIII in the MVs (94). In addition to brain tumors, EV protein cargoes, including carbonic anhydrase 9, annexin II, myoferlin, and WNT4, have been reported to enhance angiogenesis for a number of other tumor types (95–98). Compared to angiogenesis, lymphangiogenesis is relatively understudied. However, the lymphatic mucin podoplanin was shown to be incorporated into both MVs and exosomes released from HNSCC cells and to promote lymphatic vessel formation (99). This lymphangiogenesis is related to podoplanin-mediated regulation of Rho signaling (100). Interestingly, few studies have identified VEGF itself as a key EV cargo promoting angiogenesis, although some studies reported that VEGF is carried together with other angiogenic components in EVs (101–104). Instead it appears that EVs may synergize with the well-established role of soluble secreted VEGF family members by carrying additional angiogenic cargoes.
In addition to carrying proteins, EVs carry a number of extracellular RNAs. Of these, miRNAs have been the best studied and a number of these have been suggested to be involved in promoting tumor angiogenesis. An initial report that provided direct evidence of EV-mediated proangiogenic miRNA delivery showed that leukemia-derived exosomes deliver miR-92a into endothelial cells and promote angiogenesis (105). Exosomes purified from leukemia cells transfected with Cy3-labeled miR-92a were taken up by endothelial cells. The miR-92a-containing exosomes enhanced endothelial cell migration and tube formation compared with control exosomes. miR-92a has been shown to induce angiogenesis by downregulation of a5 integrin in endothelial cells (106), although in some cases miR-92a can also inhibit angiogenesis (107, 108). Exosomal transfer of miR-135b has also been shown to promote endothelial tube formation (109). miR-135b is known to promote angiogenesis by suppressing Factor Inhibiting Hypoxia inducible factor-1 (FIH-1) (110). Exosomes purified from hypoxic multiple myeloma cells decreased FIH-1 expression in endothelial cells and increased endothelial cell tube formation. The biologic effects of the purified exosomes were inhibited by expression of anti-miR-135b and augmented by overexpression of a miR-135b mimic in the parental cells. A number of other miRNAs have also been implicated in regulating tumor angiogenesis via exosomal transfer, although in many cases direct causality has not been shown (89, 111–117).
EVs have also been shown to increase vascular permeability. Similar to angiogenesis, both protein and RNA cargoes have been shown to promote vascular leakiness. Schillaci reported that metastatic tumor-derived exosomes promoted endothelial permeability more than non-metastatic tumor-derived exosomes (118). They also showed that metastatic tumor-derived exosomes were enriched for activators of RhoA/ROCK signaling, which may destabilize endothelial junctions. Co-treatment of endothelial cells with tumor-derived exosomes and a ROCK inhibitor reverted the stability of endothelial cell-cell junctions. In lung cancer, delivery of exosomal miR-23a to endothelial cells inhibited expression of the tight junction protein ZO-1, thereby increasing vascular permeability and cancer transendothelial migration (112).
Remodeling of target organs to facilitate engraftment and development of metastases.
After arrival of cancer cells in target organs, successful engraftment and growth of cancer cells requires the appropriate microenvironment. As with their role in enhancing survival at primary sites, EVs also modify microenvironments in distant organs to prepare “metastatic niches” (28, 119, 120).
The “premetastatic niche” was originally defined by David Lyden’s laboratory, in a landmark paper showing that secreted factors from melanoma cells could select sites of metastasis (e.g. the lung) in part by recruiting VEGFR1+-producing bone marrow-derived cells to those sites as well as induction of FN deposition by stromal cells (121). Subsequently, the mystery factors secreted from the melanoma cells were demonstrated to be exosomes (122). Exosomes from highly metastatic melanomas educated bone marrow-derived cells (BMDCs) toward a pro-vasculogenic and pro-metastatic phenotype through transfer of the receptor tyrosine kinase protein MET. Reducing Met expression in exosomes diminished the pro-metastatic behavior of bone marrow cells. Inhibition of exosome secretion via Rab27a RNA interference prevented bone marrow education and reduced tumor growth and metastasis. In another organ system, the same group showed that pancreatic cancer-derived exosomes induce liver pre-metastatic niche formation in naive mice and consequently increase liver metastatic burden (123). Uptake of pancreatic cancer-derived exosomes by Kupffer cells caused transforming growth factor β (TGF β) secretion and upregulation of fibronectin production by hepatic stellate cells, which enhanced metastasis.
In addition to enhancing tumor growth and engraftment at distant sites, exosomes appear to select the specific location of tumor metastasis. That is, where the exosomes lodge, so do the tumors – in an updated version of the seed-soil hypothesis (124). The cargo on EVs is likely important for the selective interaction with target cells and organs via ligand-receptor interactions. Indeed, for metastatic niche selection, specific integrins expressed on tumor-derived exosomes were shown to correlate with organ-specific metastasis (125). Exosomes from pancreatic cancer cell lines that metastasize primarily to the liver expressed αvβ5 integrin and colocalized with Kupffer cells in mouse livers after in vivo injection. Likewise, exosomes from human breast cancer cell lines that metastasize primarily to the lung expressed α6β4 and α6β1 integrins, and co-localized with S100A4-positive fibroblasts and surfactant protein C (SPC)-positive epithelial cells. KD of integrin β5 or β4 led to reduced uptake of the KD exosomes by the liver and lung, respectively, and integrin β4-KD exosomes were unable to promote metastatic seeding of breast cancer cells to the lung. Since integrins are ECM receptors, these data suggest that the exosomes are either binding to ECM at the metastatic sites or carrying ECM that then mediates binding to cognate receptors on host organ cells. Future studies are likely to define these mechanisms in more detail and define whether other integrins are involved in metastatic niche preparation.
Additional groups have fleshed out diverse mechanisms by which cancer-derived EVs enhance metastatic niche formation. CD105+ EVs secreted from renal cancer stem cells were shown to promote blood vessel-rich premetastatic niches, potentially by transfer of proangiogenic miRNAs and mRNAs (126). miR-122-carrying breast cancer EVs were shown to promote premetastatic niche formation in the brain and lung by modulating glucose metabolism in brain astrocytes and lung fibroblasts (127). Interestingly, miR-122 is a predictive marker of metastasis in breast cancer patients (128) and secreted from breast cancer. The increased metastasis of breast cancer cells induced by miR-122-overexpressing EVs was reduced by inhibition of miR-122 in the parent cells (127). Tumor-derived EVs can also drive bone metastatic niche formation. Amphiregulin (AREG) carried on non-small cell lung cancer (NSCLC) exosomes was shown to induce EGFR pathway activation in pre-osteoclasts that in turn causes increased expression of RANKL and differentiation into osteoclasts, triggering the so-called “vicious cycle” of osteolytic bone metastasis whereby factors released from bone destruction enhance tumor growth (129, 130).
Lymph nodes are also a major site of cancer metastasis. In sentinel lymph nodes, melanoma exosomes were shown to enhance seeding of melanoma cells, potentially by increasing gene expression in the lymph nodes of multiple molecular factors that promote melanoma cell recruitment, ECM deposition, and vascular proliferation (131). In another study, pancreatic exosomes were shown to seed lymph nodes and the lung dependent on exosomal expression of CD44 (132).
On the other hand, EVs can play an inhibitory role in metastasis. For example, exosomes from nonmetastatic melanoma cells can inhibit metastasis of aggressive melanoma cells by inducing immune surveillance at metastatic sites (133). Compared with exosomes purified from isogenic metastatic cells, exosomes purified from nonmetastatic cells induced expansion of patrolling monocytes (133), which are CD45+, Ly6Clow, and CX3CR1+ cells that act in the microvasculature to scavenge debris and reduce inflammation (134, 135). Patrolling monocytes had previously been found to limit metastasis to the lung by recruiting and activating NK cells and to “scavenge” EVs (136, 137). The exosomes from nonmetastatic melanoma cells also induced differentiation of macrophages to an M1 phenotype, and activation of NK cells. Pigment epithelium derived factor (PEDF) on the melanoma exosomes was found to be a key molecular cargo, both altering immune phenotypes to inhibit metastasis in mice and correlating with human melanoma patient survival. Of note, exosomes from nonmetastatic cells that were knocked-down for PEDF were much less potent in their ability to inhibit metastasis than control exosomes from the same cells. An anti-PEDF antibody also reversed the effect of “nonmetastatic” exosomes on induction of immune cytokine expression by RAW 264.7 macrophages. Interestingly, exosomes isolated from nonmetastatic patient serum inhibited experimental metastasis in mouse models, whereas exosomes isolated from metastatic patient serum had the opposite effect (133).
In addition to cancer-derived EVs, EVs released from host cells at metastatic sites also regulate establishment of tumor metastasis. In the brain, astrocyte-derived exosomes transfer PTEN-targeting miR-19a to metastatic tumor cells (138). The resultant PTEN loss leads to increased secretion of the chemokine CCL2, which in turn recruits myeloid cells that enhance the outgrowth of brain metastatic tumor cells. In the bone marrow, mesenchymal stem cell (BM-MSC)-derived exosomes were shown to promote dormancy of metastasized breast cancer cells (139). Breast cancer cells treated with BM-MSC exosomes had a dormant phenotype including a low proliferation rate and chemotherapy-resistance. From the miRNAs increased in BM-MSC exosomes compared to adult fibroblast exosomes, miR-23b was identified and its overexpression in breast cancer cells was demonstrated to induce dormancy phenotypes including downregulation of CD44 expression, enhancement of dormancy gene expression, and decreased in vivo proliferation.
From these reports, it is clear that EVs can influence the microenvironment at future metastatic sites and in many cases promote organ-specific metastasis.
Regulation of tumor immunity
The immune system is a powerful regulator of tumor progression (140). To escape this regulation and survive, tumor cells have evolved diverse mechanisms, including some that utilize EVs (30–32, 141, 142). In this section, we discuss the role of EVs in regulating diverse immune cell types, including anti-tumor cytotoxic cells and immune regulatory cells, and their potential role in modulating the response to immune checkpoint blockade therapy.
Role of EVs in cytotoxic lymphocyte regulation
CD8+ cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are major cytotoxic immune cells that target and kill cancer cells (140, 143–147). The presence of these cells in tumors is associated with good prognosis in a number of tumor types (148). Tumor-specific CD8+ CTLs and NK cells eliminate tumor cells through the production of apoptosis-inducing molecules or cytotoxic granules, such as granzyme and perforin (144, 147). Though the field is still new, a number of studies have reported that tumor-derived EVs can directly affect cytotoxic function, proliferation and survival of CD8+ CTLs and NK cells (30, 32, 149–157). Early studies showed that EVs carrying Fas ligand (FasL) could induce apoptosis of CD8+ CTLs (149–151, 153, 154), due to their expression of the FasL receptor CD95. TRAIL expressed on tumor exosomes may also contribute to induction of CD8 CTL apoptosis (150). More recently, tumor-derived EVs have been shown to downregulate the activation status of NK and CD8+ T cells by downregulating the receptor NKG2D (152, 155–157). NKG2D is a lectin-like activating receptor that is involved in recognition of transformed and stressed cells and can also activate NK and CTL cytotoxicity, in part by inducing release of cytokines (158, 159). Exosomes from various cancer cell lines or isolated from pleural effusions of mesothelioma patients carry several NKG2D ligands, including MHC class I–related chain (MIC) A (MICA), MICA *008, MICB or UL16-binding proteins 2 (ULBP2), as well as TGFb, which induce downregulation of NKG2D surface levels (155–157). This downregulation appears to affect function, as NKG2D-agonist ab-induced lymphocyte cytotoxicity and secretion of interferon-g was reduced in exosome-treated cells (156). Tumor derived exosomes containing MICA *0008 have also been shown to downregulate “bystander” cell killing by NK cells, presumably still via downregulation of NKG2D with the accompanying reduction of lymphocyte activation (157). In that study, both NKG2D- and non-NKG2D-dependent cell killing were shown to be attenuated by tumor-derived exosome treatment.
Control of Regulatory Immune Cells by EVs
EVs have been shown to regulate additional types of immune cells, including dendritic cells (DCs), regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs)(160–171). These cells are critical for orchestrating anticancer cytotoxicity, and dysfunction of these cells is common in cancer (172–174). Although tumor-derived EVs carry tumor antigens to DCs and activate tumor-specific CTLs (163, 164), they can also suppress DC functions. Breast cancer cell-derived exosomes were found to block the maturation of DCs from precursor cells, by delivering IL-6 to activate STAT3 signaling (165). Similarly, tumor-derived EVs were found to interfere with normal differentiation of DCs (166). Monocytes treated with tumor-derived EVs secreted TGFb which suppressed T cell proliferation and cytolytic ability; however detailed mechanisms of this tumor derived MV-mediated phenotypic change are as of yet unknown. Tumor-derived EVs may also regulate function of DCs via activation of Toll-like receptors (TLRs)-Type I interferon (IFN) pathway (167). Downregulation of the Hippo pathway kinases LATS1/2 in tumor cells led to enrichment of nucleic acids in EVs which activated the TLR-Type I IFN pathway in DCs and enhanced secretion of IL-12 to promote cytotoxic activity of CD8+ CTLs.
Tregs control immune responses to foreign antigens including tumor-derived ones, and play a role in preventing tumor rejection (175). Increased frequencies of tumor Tregs correlate with poor prognosis of cancer patients, probably as a consequence of Treg-mediated suppression of antitumor immunity (176). Ovarian cancer- and HNSCC-derived EVs have been shown to induce promote differentiation of CD3+CD4+ T cells into CD4+CD25highFOXP3+ Tregs (168). The EVs also promoted expansion and immunosuppressive functions of Treg cells via upregulation of FasL, TGFb, IL-10, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), granzyme B and perforin. In another study, nasopharyngeal carcinoma (NPC)-derived exosomes were similarly found to recruit conventional CD4 T cells and induce their conversion into suppressive Treg cells (161).
Along with Tregs, MDSCs are major immune suppressive cells in the tumor microenvironment (TME) (173). Many studies have reported that tumor-derived EVs promote conversion of monocytes to MDSCs (160, 166, 169–171). The mechanisms include activation of TLR and STAT3 pathways by HSP70 family members expressed on the surface of exosomes. The exosomal HSP70 and HSP72 were shown to interact with TLR2 to promote IL6 secretion and STAT3 phosphorylation (160, 171)
Role of EVs in Immune Checkpoint Control
Recently, immune checkpoint blockade (ICB) has taken on an important role in cancer therapy (177–179). Tumor cells evade killing by immune cells by activating immune checkpoint molecules, which serve to downregulate immune responses via ligand-receptor interactions between T cells, dendritic cells, tumor cells, and macrophages (177, 180). The major and well-studied immune checkpoint molecules are programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and CTLA-4 (180–182). PD-1 is expressed on activated T cells. Activation of PD-1 by either of its two ligands, PD-L1 and PD-L2, downregulates signaling associated with antigen recognition by the T cell receptor. PD-L2 is predominantly expressed on antigen-presenting cells (APCs) whereas PD-L1 can be expressed on many cell types, including cancer cells. CTLA-4 is also expressed by activated T-cells and downregulates T-cell function through a variety of mechanisms, including preventing costimulation by CD28 and inducing cell cycle arrest (181, 182). EVs released from chronic lymphocytic leukemia (CLL) cells have been shown to regulate expression of PD-L1 expression in monocytes (183). From the results of RNA sequencing and proteome analyses, noncoding Y RNA hY4 was enriched in exosomes from the plasma of CLL patients compared with healthy donor samples. Transfer of CLL-derived exosomes or hY4 alone to monocytes increased the expression of PD-L1, attenuating tumor immunity to support cancer progression. In other cancers, tumor-derived EVs were shown to increased expression of cytotoxic T lymphocyte antigen 4 (CTLA-4) in Tregs (168), which may enhance Treg activity.
From these studies, some specific immune cell-directed effects of EVs have been identified. However, the immune system is highly complex, and more comprehensive and in-depth studies will be needed to understand the role of EVs in coordinating and controlling anti-tumor immunity.
Conclusions
Many functions of EVs in cancer progression have been identified in the past few years, including roles in promoting cancer invasiveness and metastasis, angiogenesis, and immune regulation. In some cases, molecular mechanisms have been identified; however, by-and-large the role of EV molecular cargoes in various functions remains to be determined. Therefore, a major focus for the future should be on designing clear experiments to definitively test the role of individual or multiple EV cargoes on biological functions. For example, engineered loss or gain of specific cargoes should lead to loss or gain of EV activities in functional assays. Ideally, one should also check whether those perturbations affect additional cargoes in EVs. This is a difficult but important task and must take place before EV therapies targeting specific cargoes can be designed. In addition, many studies focus somewhat arbitrarily on only one class of cargo, e.g. RNA, protein, or lipid, which may limit the scope of investigation and lead to missing important molecular mechanisms. A more broad-based approach may lead to important mechanistic insights. Careful purification and characterization of EVs, such as has been proposed in several publications (184, 185), are also an important component of increasing the rigor and reproducibility of EV experiments and identifying bona fide EV cargoes responsible for biological functions. Finally - although rarely reported to date - it also is the case that sometimes EV secretion from cancer cells may inhibit cancer progression, for example from relatively nonaggressive tumors (133). Understanding which patients to choose for anti-EV therapy, such as drugs that inhibit exosome secretion, should also take such considerations into account. Overall, it is clear that EVs are a major component of the tumor microenvironment that orchestrates the interaction between cancer and host cells to drive cancer progression.
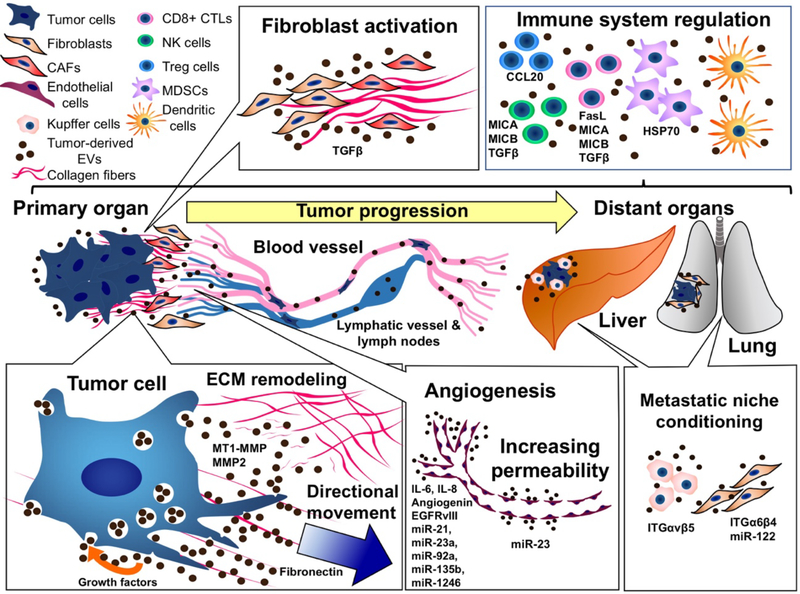
Figure 1. The role of extracellular vesicles (EVs) in tumor progression.
Tumor cell-derived EVs mediate both paracrine and autocrine communication. At the primary lesion, tumor-derived EVs facilitate autocrine mechanisms of growth, migration and invasion via growth factor, ECM and proteinase cargoes. Paracrine communication via cancer-derived EVs promotes transformation of fibroblasts into cancer-associated fibroblasts (CAFs) that can also promote cancer invasiveness via remodeling of ECM. Tumor-derived EVs also facilitate angiogenesis and promote vascular permeability, which may promote intravasation of tumor cells. Tumor-derived EVs can reach distant organs and affect organ-specific stromal cells, such as Kupffer cells in the liver or fibroblasts in the lung, to promote pre-metastatic niche formation. Tumor immunity can also be influenced by cancer-derived EVs, including attenuation of cytotoxic function of CD8+ CTLs and NK cells, and increased activity of Treg cells and MDSCs. EV molecular cargoes shown in the figure are from references (46, 47, 66, 92–94, 105, 109, 111, 112, 125, 127, 151–157, 160, 161, 171).
Summary Points.
Extracellular vesicles (EVs) constitute a major mechanism of cellular communication.
Tumor-derived EVs play important roles in multiple steps of cancer progression, including cancer invasiveness and metastasis, angiogenesis, and immune regulation.
In the process of cancer progression, tumor-derived EVs can act in both an autocrine and paracrine manner.
The role of EV molecular cargoes in various functions is still poorly defined and is an important area of future investigation.
References
- 1.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60(4):834–40. [PubMed] [Google Scholar]
- 3.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78. [DOI] [PubMed] [Google Scholar]
- 4.Campos JH, Soares RP, Ribeiro K, Andrade AC, Batista WL, Torrecilhas AC. Extracellular Vesicles: Role in Inflammatory Responses and Potential Uses in Vaccination in Cancer and Infectious Diseases. J Immunol Res. 2015;2015:832057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. [DOI] [PubMed] [Google Scholar]
- 6.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80(6):1948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55. [DOI] [PubMed] [Google Scholar]
- 9.Marcilla A, Martin-Jaular L, Trelis M, de Menezes-Neto A, Osuna A, Bernal D, et al. Extracellular vesicles in parasitic diseases. J Extracell Vesicles. 2014;3:25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coakley G, McCaskill JL, Borger JG, Simbari F, Robertson E, Millar M, et al. Extracellular Vesicles from a Helminth Parasite Suppress Macrophage Activation and Constitute an Effective Vaccine for Protective Immunity. Cell Rep. 2017;19(8):1545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20. [PubMed] [Google Scholar]
- 12.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 13.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–20. [DOI] [PubMed] [Google Scholar]
- 14.Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319–23. [DOI] [PubMed] [Google Scholar]
- 16.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109(11):4146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider DJ, Speth JM, Penke LR, Wettlaufer SH, Swanson JA, Peters-Golden M. Mechanisms and modulation of microvesicle uptake in a model of alveolar cell communication. J Biol Chem. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–8. [DOI] [PubMed] [Google Scholar]
- 19.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69(13):5601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meehan B, Rak J, Di Vizio D. Oncosomes - large and small: what are they, where they came from? J Extracell Vesicles. 2016;5:33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin Cell Dev Biol. 2017;67:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan S, Danielson S, Clements V, Edwards N, Ostrand-Rosenberg S, Fenselau C. Surface Glycoproteins of Exosomes Shed by Myeloid-Derived Suppressor Cells Contribute to Function. J Proteome Res. 2017;16(1):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stec M, Szatanek R, Baj-Krzyworzeka M, Baran J, Zembala M, Barbasz J, et al. Interactions of tumour-derived micro(nano)vesicles with human gastric cancer cells. J Transl Med. 2015;13:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164(6):1226–32. [DOI] [PubMed] [Google Scholar]
- 26.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30(6):836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maas SL, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27(3):172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93. [DOI] [PubMed] [Google Scholar]
- 32.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalluri R The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dozio V, Sanchez JC. Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J Extracell Vesicles. 2017;6(1):1302705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Z, Batagov AO, Schinelli S, Wang J, Wang Y, El Fatimy R, et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8(1):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. [DOI] [PubMed] [Google Scholar]
- 40.Paz H, Pathak N, Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene. 2014;33(33):4193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105(5):1211–8. [DOI] [PubMed] [Google Scholar]
- 42.Laghezza Masci V, Taddei AR, Gambellini G, Giorgi F, Fausto AM. Microvesicles shed from fibroblasts act as metalloproteinase carriers in a 3-D collagen matrix. J Circ Biomark. 2016;5:1849454416663660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clancy JW, Sedgwick A, Rosse C, Muralidharan-Chari V, Raposo G, Method M, et al. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat Commun. 2015;6:6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C, et al. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One. 2013;8(9):e75054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214(2):197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM, et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49(8):1845–59. [DOI] [PubMed] [Google Scholar]
- 51.Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. 2016;6:38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felicetti F, Parolini I, Bottero L, Fecchi K, Errico MC, Raggi C, et al. Caveolin-1 tumor-promoting role in human melanoma. Int J Cancer. 2009;125(7):1514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69(3):785–93. [DOI] [PubMed] [Google Scholar]
- 54.Majumdar R, Tavakoli Tameh A, Parent CA. Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis. PLoS Biol. 2016;14(1):e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108(12):4852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290(8):4545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol. 2008;18(12):926–31. [DOI] [PubMed] [Google Scholar]
- 59.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung BH, Weaver AM. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr. 2017;11(2):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sedgwick AE, Clancy JW, Olivia Balmert M, D’Souza-Schorey C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci Rep. 2015;5:14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160(2):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hager MH, Morley S, Bielenberg DR, Gao S, Morello M, Holcomb IN, et al. DIAPH3 governs the cellular transition to the amoeboid tumour phenotype. EMBO Mol Med. 2012;4(8):743–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paluch EK, Raz E. The role and regulation of blebs in cell migration. Curr Opin Cell Biol. 2013;25(5):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. [DOI] [PubMed] [Google Scholar]
- 67.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–30. [DOI] [PubMed] [Google Scholar]
- 68.Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D’Ippolito E, Cataldo A, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7(7):e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gopal S, Veracini L, Grall D, Butori C, Schaub S, Audebert S, et al. Fibronectin-guided migration of carcinoma collectives. Nat Commun. 2017;8:14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216(11):3799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanisavljevic J, Loubat-Casanovas J, Herrera M, Luque T, Pena R, Lluch A, et al. Snail1-expressing fibroblasts in the tumor microenvironment display mechanical properties that support metastasis. Cancer Res. 2015;75(2):284–95. [DOI] [PubMed] [Google Scholar]
- 73.Riching KM, Cox BL, Salick MR, Pehlke C, Riching AS, Ponik SM, et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys J. 2014;107(11):2546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, et al. Cancer-associated fibroblasts lead tumor invasion through integrin-beta3-dependent fibronectin assembly. J Cell Biol. 2017;216(11):3509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pankova D, Chen Y, Terajima M, Schliekelman MJ, Baird BN, Fahrenholtz M, et al. Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma. Mol Cancer Res. 2016;14(3):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimoda M, Principe S, Jackson HW, Luga V, Fang H, Molyneux SD, et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat Cell Biol. 2014;16(9):889–901. [DOI] [PubMed] [Google Scholar]
- 77.Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian H, et al. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Rep. 2016;14(4):3452–8. [DOI] [PubMed] [Google Scholar]
- 78.Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8(12):19592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casadei L, Calore F, Creighton CJ, Guescini M, Batte K, Iwenofu OH, et al. Exosome-Derived miR-25–3p and miR-92a-3p Stimulate Liposarcoma Progression. Cancer Res. 2017;77(14):3846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E, et al. Exosomes Promote Ovarian Cancer Cell Invasion through Transfer of CD44 to Peritoneal Mesothelial Cells. Mol Cancer Res. 2017;15(1):78–92. [DOI] [PubMed] [Google Scholar]
- 82.Silva AM, Almeida MI, Teixeira JH, Maia AF, Calin GA, Barbosa MA, et al. Dendritic Cell-derived Extracellular Vesicles mediate Mesenchymal Stem/Stromal Cell recruitment. Sci Rep. 2017;7(1):1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, LeGonidec S, et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016;76(14):4051–7. [DOI] [PubMed] [Google Scholar]
- 84.Gernapudi R, Yao Y, Zhang Y, Wolfson B, Roy S, Duru N, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat. 2015;150(3):685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–60. [DOI] [PubMed] [Google Scholar]
- 86.Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, et al. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74. [DOI] [PubMed] [Google Scholar]
- 89.Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes Function in Pro- and Anti-Angiogenesis. Curr Angiogenes. 2013;2(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su SA, Xie Y, Fu Z, Wang Y, Wang JA, Xiang M. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget. 2017;8(15):25700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ Res. 2017;120(10):1658–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–24. [DOI] [PubMed] [Google Scholar]
- 95.Horie K, Kawakami K, Fujita Y, Sugaya M, Kameyama K, Mizutani K, et al. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun. 2017;492(3):356–61. [DOI] [PubMed] [Google Scholar]
- 96.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, et al. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol Cancer Res. 2017;15(1):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blomme A, Fahmy K, Peulen O, Costanza B, Fontaine M, Struman I, et al. Myoferlin is a novel exosomal protein and functional regulator of cancer-derived exosomes. Oncotarget. 2016;7(50):83669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang Z, Feng Y. Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced beta-Catenin Signaling in Endothelial Cells. Oncol Res. 2017;25(5):651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carrasco-Ramirez P, Greening DW, Andres G, Gopal SK, Martin-Villar E, Renart J, et al. Podoplanin is a component of extracellular vesicles that reprograms cell-derived exosomal proteins and modulates lymphatic vessel formation. Oncotarget. 2016;7(13):16070–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Navarro A, Perez RE, Rezaiekhaligh M, Mabry SM, Ekekezie, II. T1alpha/podoplanin is essential for capillary morphogenesis in lymphatic endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L543–51. [DOI] [PubMed] [Google Scholar]
- 101.Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. 2017;6(1):1359479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun. 2017;8:14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song YH, Warncke C, Choi SJ, Choi S, Chiou AE, Ling L, et al. Breast cancer-derived extracellular vesicles stimulate myofibroblast differentiation and pro-angiogenic behavior of adipose stem cells. Matrix Biol. 2017;60–61:190–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giusti I, Delle Monache S, Di Francesco M, Sanita P, D’Ascenzo S, Gravina GL, et al. From glioblastoma to endothelial cells through extracellular vesicles: messages for angiogenesis. Tumour Biol. 2016;37(9):12743–53. [DOI] [PubMed] [Google Scholar]
- 105.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–55. [DOI] [PubMed] [Google Scholar]
- 106.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–3. [DOI] [PubMed] [Google Scholar]
- 107.Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103(4):564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murata K, Ito H, Yoshitomi H, Yamamoto K, Fukuda A, Yoshikawa J, et al. Inhibition of miR-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J Bone Miner Res. 2014;29(2):316–26. [DOI] [PubMed] [Google Scholar]
- 109.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86(2):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y, et al. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta. 2014;1839(11):1256–72. [DOI] [PubMed] [Google Scholar]
- 112.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–42. [DOI] [PubMed] [Google Scholar]
- 113.Kinoshita T, Yip KW, Spence T, Liu FF. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet. 2017;62(1):67–74. [DOI] [PubMed] [Google Scholar]
- 114.Ghayad SE, Rammal G, Ghamloush F, Basma H, Nasr R, Diab-Assaf M, et al. Exosomes derived from embryonal and alveolar rhabdomyosarcoma carry differential miRNA cargo and promote invasion of recipient fibroblasts. Sci Rep. 2016;6:37088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288(48):34343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lawson J, Dickman C, MacLellan S, Towle R, Jabalee J, Lam S, et al. Selective secretion of microRNAs from lung cancer cells via extracellular vesicles promotes CAMK1D-mediated tube formation in endothelial cells. Oncotarget. 2017;8(48):83913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sruthi TV, Edatt L, Raji GR, Kunhiraman H, Shankar SS, Shankar V, et al. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. J Cell Physiol. 2017. [DOI] [PubMed] [Google Scholar]
- 118.Schillaci O, Fontana S, Monteleone F, Taverna S, Di Bella MA, Di Vizio D, et al. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Sci Rep. 2017;7(1):4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–17. [DOI] [PubMed] [Google Scholar]
- 121.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paget S THE DISTRIBUTION OF SECONDARY GROWTHS IN CANCER OF THE BREAST. Lancet. 1889;133(3421):571–3. [PubMed] [Google Scholar]
- 125.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–56. [DOI] [PubMed] [Google Scholar]
- 127.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu X, Somlo G, Yu Y, Palomares MR, Li AX, Zhou W, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med. 2012;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taverna S, Pucci M, Giallombardo M, Di Bella MA, Santarpia M, Reclusa P, et al. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep. 2017;7(1):3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93. [DOI] [PubMed] [Google Scholar]
- 131.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–801. [DOI] [PubMed] [Google Scholar]
- 132.Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15(3):281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun. 2017;8(1):1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cassetta L, Pollard JW. Cancer immunosurveillance: role of patrolling monocytes. Cell Res. 2016;26(1):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buscher K, Marcovecchio P, Hedrick CC, Ley K. Patrolling Mechanics of Non-Classical Monocytes in Vascular Inflammation. Front Cardiovasc Med. 2017;4:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350(6263):985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kubo H, Mensurado S, Goncalves-Sousa N, Serre K, Silva-Santos B. Primary Tumors Limit Metastasis Formation through Induction of IL15-Mediated Cross-Talk between Patrolling Monocytes and NK Cells. Cancer Immunol Res. 2017;5(9):812–20. [DOI] [PubMed] [Google Scholar]
- 138.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. [DOI] [PubMed] [Google Scholar]
- 140.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30. [DOI] [PubMed] [Google Scholar]
- 142.Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44(6):1255–69. [DOI] [PubMed] [Google Scholar]
- 143.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. [DOI] [PubMed] [Google Scholar]
- 144.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–36. [DOI] [PubMed] [Google Scholar]
- 145.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. [DOI] [PubMed] [Google Scholar]
- 147.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. [DOI] [PubMed] [Google Scholar]
- 149.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9(14):5113–9. [PubMed] [Google Scholar]
- 150.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128(7):1796–804. [DOI] [PubMed] [Google Scholar]
- 151.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195(10):1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lundholm M, Schroder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9(9):e108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11(3):1010–20. [PubMed] [Google Scholar]
- 154.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35(2):169–73. [DOI] [PubMed] [Google Scholar]
- 155.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34(3):206–13. [DOI] [PubMed] [Google Scholar]
- 156.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180(11):7249–58. [DOI] [PubMed] [Google Scholar]
- 157.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res. 2015;3(6):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015;107(1):363. [DOI] [PubMed] [Google Scholar]
- 162.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. [DOI] [PubMed] [Google Scholar]
- 163.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. [DOI] [PubMed] [Google Scholar]
- 164.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. [DOI] [PubMed] [Google Scholar]
- 165.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–75. [DOI] [PubMed] [Google Scholar]
- 166.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66(18):9290–8. [DOI] [PubMed] [Google Scholar]
- 167.Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, et al. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell. 2016;167(6):1525–39 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010;5(7):e11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiang X, Liu Y, Zhuang X, Zhang S, Michalek S, Taylor DD, et al. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol. 2010;177(4):1606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A, et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes With a HSP70 Peptide Aptamer. J Natl Cancer Inst. 2016;108(3). [DOI] [PubMed] [Google Scholar]
- 172.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–83. [DOI] [PubMed] [Google Scholar]
- 173.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. [DOI] [PubMed] [Google Scholar]
- 175.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16(4):220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18(11):3022–9. [DOI] [PubMed] [Google Scholar]
- 177.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–84. [DOI] [PubMed] [Google Scholar]
- 179.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. [DOI] [PubMed] [Google Scholar]
- 180.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14(11):655–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Haderk F, Schulz R, Iskar M, Cid LL, Worst T, Willmund KV, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol. 2017;2(13). [DOI] [PubMed] [Google Scholar]
- 184.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228–32. [DOI] [PubMed] [Google Scholar]