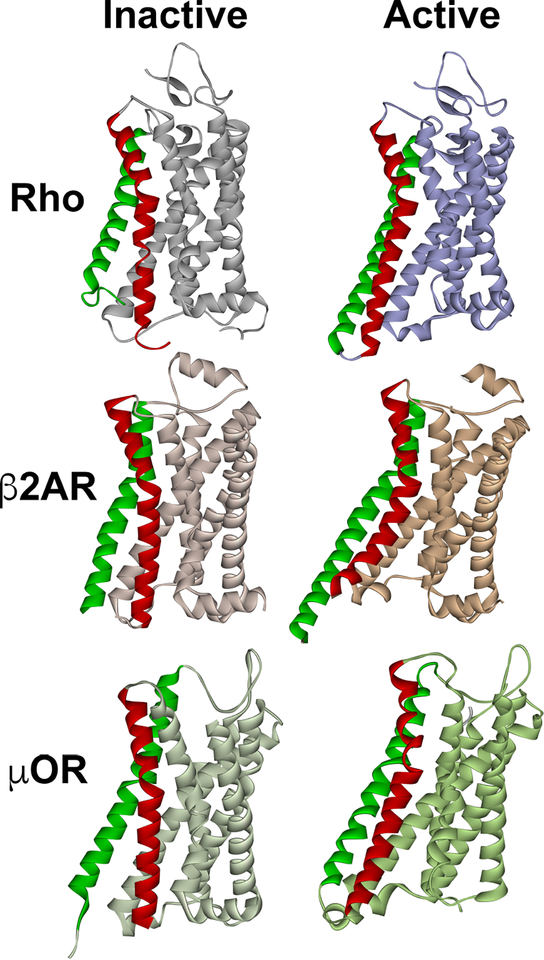
Fig. 1. The structure of inactive and active GPCRs.
The most pronounced activation-induced conformational change in all GPCRs is the outward movement of TM5 (green) and TM6 (red). In case of TM6, activation-induced shift is ∼ 14 A in Gs-coupled β2AR (Rasmussen et al., 2011), but smaller in Gi-coupled receptors, ∼10 A in both rhodopsin (Kang et al., 2018; Kang et al., 2015) and μ-opioid receptor (Koehl et al., 2018). The structures of inactive (dark) rhodopsin (Rho) (PDB ID: 1GZM (Li et al., 2004)), active rhodopsin (from complex with arrestin-1; PDB ID: 4ZWJ (Kang et al., 2015)), inactive β2AR (PDB ID: 2RH1 (Rasmussen et al., 2007)), active β2AR (from complex with Gs; PDB ID: 3SN6 (Rasmussen et al., 2011)), inactive μ-opioid receptor (μOR) (PDB ID: 4DKL (Manglik et al., 2012)), and active μOR (from complex with Gi; PDB ID: 6DDE (Koehl et al., 2018)) are shown. The common feature of active GPCR structures is the cavity between helices that opens on the cytoplasmic side. In case of Gi-coupled GPCRs (Rho and μOR) the outward movement of the helices is smaller, so that this cavity is narrower than in case of Gs-coupled β2AR. The dimensions of this cavity might underlie GPCR selectivity for particular G proteins, whereas non-visual arrestins likely accommodate GPCRs that couple to all types of G proteins.