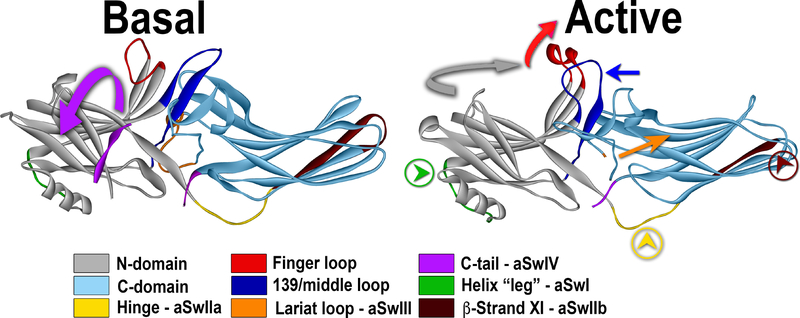
Fig. 5. Activation of arrestin-3 and switch regions.
Conformational changes upon arrestin-3 activation are shown: the release of the arrestin C-terminus (magenta; magenta arrow shows the direction of the movement; aSwIV) anchored to the body of the molecule via the three-element interaction in the basal state (PDB ID: 3P2D (Zhan et al., 2011a)), the movement of the finger loop and helix formation in its tip (red; red arrow shows the direction of the movement), the twist of the two domains relative to each other (N-domain, gray; C-domain, teal). Movements of several loops, likely creating effector docking sites, are also shown: middle loop (dark blue; dark blue arrow indicates the direction of movement); the “leg” of the α-helix I (green, with green arrowhead in a circle; aSwI), inter-domain hinge (yellow with yellow arrowhead in a circle; aSwIIa), register-shifted β-strand XI in the C-domain (dark brown; aSwIIb), and the lariat loop (orange with orange arrow; in the active structure (PDB ID: 5TV1 (Chen et al., 2017)) it is not resolved; aSwIII). All arrestin subtypes have switches II, III, and IV, whereas only arrestin-1 has polyproline motifs in the switch I. Note that only the interactions of clathrin and AP2 with the aSwIV were experimentally confirmed so far (Kim and Benovic, 2002).