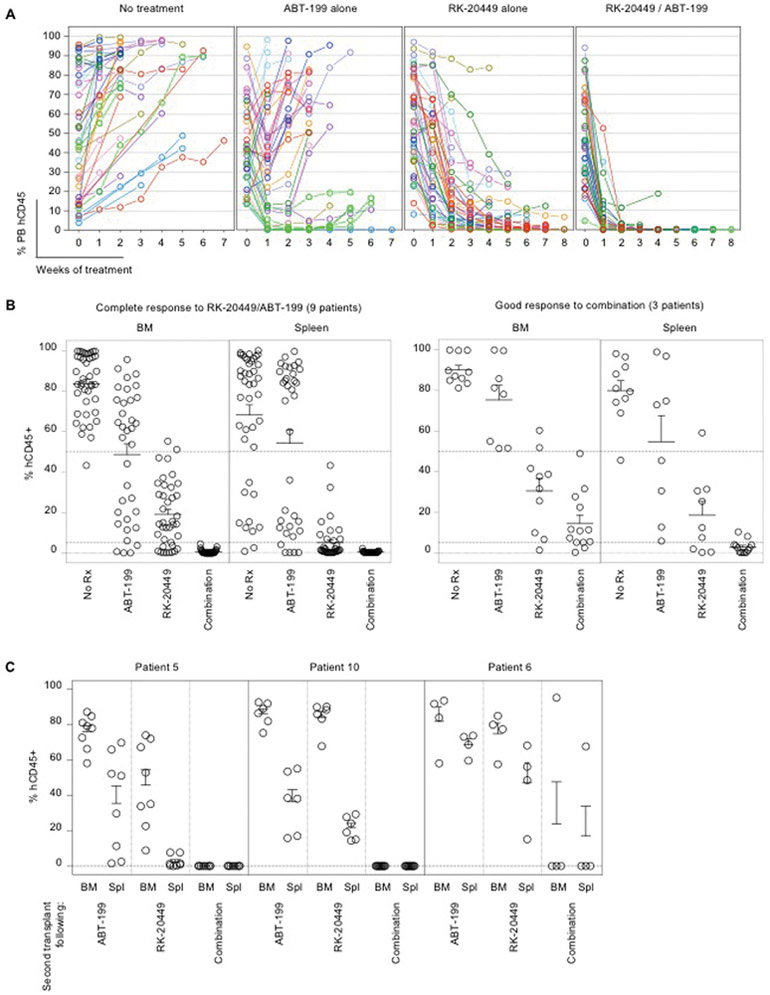
Figure 6.
Eradication of FLT3-ITD+ AML cells in vivo through combined inhibition of kinase and anti-apoptotic pathways
(A,B) Mice engrafted with AML derived from 12 patients associated with good or partial responses to RK-20449 alone received four different treatments (no treatment, ABT-199 alone, RK-20449 alone, combination). The numbers of recipients for each patient/each treatment group and pre-/post-treatment AML chimerism are shown in table S4. (A) PB human CD45+ chimerism is shown over time. Recipients were phlebotomized weekly, and pre-treatment PB human CD45+ AML chimerism is shown at time 0. Mean PB human CD45+ chimerism for each patient/each treatment group and statistics comparing treatment groups are shown in table S5.
(B) Final BM and spleen human AML chimerism is shown for mice engrafted with AML derived from 12 patients. 9 cases showed complete responses and 3 cases showed good responses to combination treatment. Each circle represents an AML-engrafted recipient. Mean BM/spleen human CD45+ chimerism for each patient/each treatment group and statistics comparing treatment groups are shown in table S6. In each scatter graph, dotted lines are drawn at 50%, 5%, and 0%.
(C) Residual human AML initiation capacity of human CD45+ cells after in vivo treatment was assessed by serial transplantation for four treatment groups. To compare the amount of residual LICs in recipients after in vivo treatment, each secondary recipient was transplanted with human CD45+ cells sorted from 2.5% (by cell number) of viable BM cells remaining in treated recipients. Mean and SEM for human CD45+ AML cell chimerism in the BM of secondary recipients are shown. Each circle represents a secondary recipient.