Fig. 4. Methylation of carnosine backbone diminishes its aldehyde quenching potential.
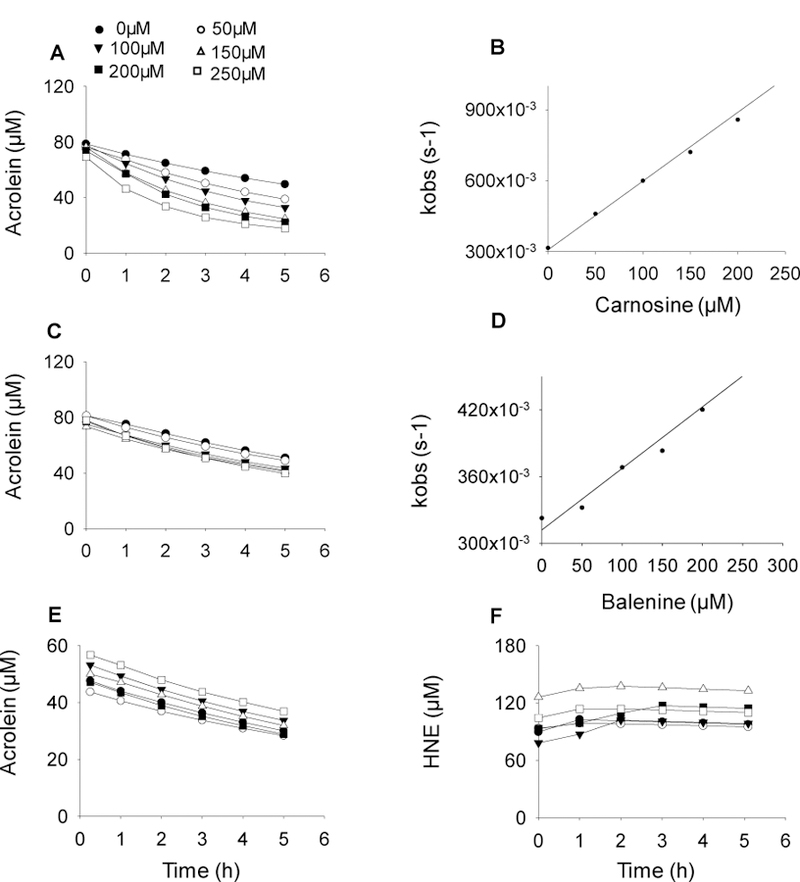
Rate of disappearance of acrolein (100 μM) in a reaction mixture containing different concentrations of (A) carnosine, (C) balenine, and (E) dimethyl balenine (50–250 µM) in 0.15M potassium phosphate buffer, pH 7.4. (F) Rate of disappearance of HNE incubated with DMB. The reaction mixtures were incubated at 37°C and the decrease in absorbance of acrolein and HNE were monitored at 215 nm and 223 nm respectively. (B, D) Data are shown as discrete points and curves were best fit of a single exponential equation to the data [y=Ae−kobs.t]. Concentration dependence of kobs. Second order rate constants were calculated from the best fits of the linear dependence and are shown in Table I.
