Abstract
Some individuals with schizophrenia report similar feelings of positive affect “in the moment” compared to control participants but report decreased trait positive affect overall. One possible explanation for this disconnection between state and trait positive affect is the extent to which individuals with schizophrenia engage in elaborative processing of positive stimuli. To assess this, we examined evoked gamma band activity in response to positive words over several seconds in a group with schizophrenia, a group with major depressive disorder, and a healthy control group. From a pre-stimulus baseline to 2000 ms after onset of the stimulus (henceforth, “early period”), the schizophrenia group showed a reliable increase in gamma activity compared to both the control and depressed groups, who did not differ from each other. In contrast, the depressed group showed a reliable increase in gamma activity from 2001 to 8000 ms (henceforth, “late period”) compared to the other groups, who did not differ from each other. At the same time, the schizophrenia group showed a reliable decrease from the early to late period while the depressed group showed the opposite pattern. In addition, self-reported depression and social anhedonia in the schizophrenia group were related to decreased gamma band activity over the entire processing window. Overall, these results suggest that schizophrenia is associated with increased initial reactivity but decreased sustained elaborative processing over time, which could be related to decreased trait positive affect. The results also highlight the importance of considering depressive symptomology and anhedonia when examining emotional abnormalities in schizophrenia.
Keywords: EEG, sustained processing, positive affect, anhedonia
1. Introduction
Schizophrenia is associated with a number of emotion-related abnormalities that are not well understood (e.g., Kohler and Martin, 2006). For example, a growing body of literature suggests that some people with schizophrenia report experiencing similar amounts of positive emotion when exposed to emotionally evocative stimuli (Cohen and Minor, 2010; Kring and Moran, 2008; Llerena et al., 2012) and in daily life (Gard et al., 2007; Myin-Germeys et al., 2000; Oorschot et al., 2009) compared to healthy individuals. At the same time, individuals with schizophrenia report substantially lower levels of trait positive affect (e.g., Horan et al., 2008; Martin et al., 2013), and when giving retrospective reports to clinicians, are rated as having less positive emotion (Strauss and Gold, 2012). Thus, there is an apparent disconnect between reports of current positive feelings (i.e., state affect) and reports of potentially noncurrent positive feelings, such as trait affect, in schizophrenia.
One possible explanation for the disconnection between state and trait positive affect in schizophrenia, which we examine in this study, is that individuals with schizophrenia have decreased, sustained elaborative processing of positive stimuli. Elaborative processing includes “embellishing” stimuli and linking them to other information (Anderson, 2005). It deepens encoding of to-be-remembered information (Anderson and Reder, 1979), which results in more durable and easily recalled memories (Anderson, 2005; Craik and Lockhart, 1972) than non-elaborated material. Thus, although individuals with schizophrenia may have intact initial reactivity to positive stimuli, their lack of sustained elaborative processing hinders encoding, consolidation, and subsequent recall of these positive feelings. Consistent with this theory, schizophrenia is associated with memory impairment for emotional experiences, particularly over long time periods (Herbener, 2008). Overall, elaborative processing of positive stimuli enhances recall of positive emotional experiences at a later time; consequently, one’s ability to engage in elaborative processing, such as savoring positive experiences, is related to emotional functioning.
In addition, particular symptoms associated with schizophrenia might be especially related to the extent to which they engage in elaborative processing. For example, decreased ability to savor positive emotions is associated with anhedonia (Applegate et al., 2009), and treatment studies focused on recalling and savoring positive emotional experiences in schizophrenia have led to decreases in anhedonia (Favrod et al., 2015; Johnson et al., 2011). Providing evidence for this theory could be important as 1) low positive affect in people with schizophrenia-spectrum disorders is not well treated with existing interventions (e.g., Grant et al., 2012) and 2) existing interventions targeting increased elaboration on positive information (e.g., McMakin et al., 2011) could be examined in this context.
In contrast to the majority of findings for schizophrenia, major depressive disorder is more consistently associated with decreased self-reported positive feelings and ratings in response to pleasant stimuli in the moment compared to healthy controls (e.g., Dunn et al., 2004; Sloan et al., 2001). In addition, individuals with major depressive disorder (Joormann and Gotlib, 2007), as well as those at risk for developing it (Joormann et al., 2007), have an increased attentional bias towards negative stimuli compared to control participants. Also, self-report and behavioral work consistently reflects poor memory for positive information (Blaney, 1986; MacLeod and Mathews, 1991; Matt et al., 1992) and decreased positive elaboration in depression (e.g., Horner et al., 2014). Thus, it is possible that depression might be associated with negatively interpreting, negatively elaborating on, or explicitly regulating responses to (Heller et al., 2015) positive stimuli. At the same time, similar to individuals with schizophrenia, actively engaging in elaborative processing, such as savoring positive emotional experiences, has been associated with improved mood in depressed samples (McMakin et al., 2011; Young et al., 2014). Thus, in this study, we compared individuals with schizophrenia to healthy individuals and individuals with depression.
Our dependent measure was evoked gamma band (35-45 Hz) electroencephalographic activity, which has been hypothesized to reflect such elaborative processes (Basar, 2013). Gamma oscillations have been argued to be the fundamental process that links neuronal activity and structural neuronal connectivity (Fries, 2009) through which higher order cognitive processes are achieved (Basar, 2013). For example, gamma band responses evoked by a stimulus are associated with crucial cognitive processes associated with elaborative processing such as feature binding (Engel and Singer, 2001), object representation (Bertrand and Tallon-Baudry, 2000), attention (Debener et al., 2003; Ray et al., 2008), and memory (e.g., Johnson and Knight, 2015; Kucewicz et al., 2014). In addition, consistent with the notion that affective information is more “attention grabbing” than non-affective stimuli (Bradley et al., 2001), gamma band activity is greater for valenced stimuli compared to neutral while passively viewing (Muller et al., 1999; Senkowski et al., 2011), and increased gamma band activity has been associated with enhanced memory for emotional information (Headley and Pare, 2013).
At the same time, a growing body of literature highlights the importance of considering earlier and later portions of the time course of emotional information processing associated with gamma band activity in schizophrenia (for a review, Basar, 2013), over which nuances have been observed. For instance, some have reported intact early, but abnormal later, gamma activity in schizophrenia in response to non-emotional stimuli while others have reported the opposite pattern (e.g., Gallinat et al., 2004; Lee et al., 2003). Thus, it is possible that individuals with schizophrenia might show intact, or even greater, gamma reactivity during the early time period because the positive emotional stimuli are attention-grabbing and novel to them, but show a significant reduction in gamma activity from the early to late period because of deficits in the ability to engage in sustained elaborative processing of positive stimuli. Similarly, if individuals with depression engage in decreased elaborative processing compared to healthy people, we would expect them to show decreased gamma activity in response to positive stimuli. However, if individuals with depression engage in negatively elaborating positive stimuli (e.g., Horner et al., 2014), we would expect them to show increased gamma activity in response to positive stimuli compared to the other groups.
In the current sample, we have previously shown that individuals with schizophrenia displayed decreased baseline gamma activity but no difference in reactivity to negative words, and participants with depression displayed increased gamma band reactivity over the course of several seconds after the presentation of negative words compared to controls (Siegle et al., 2010). The current study tested whether gamma band activity would be similar in response to positively valenced words over time in that sample.
2. Materials and Methods
Participants, methods, and analytic strategy were largely as described in Siegle et al. (2010) with the primary difference being that study concentrated on reactivity to negative words whereas the current study regarded reactivity to positive words.
2.1. Participants
The current study involved two patient groups and one healthy control group as reported in Siegle et al. (2010). The first patient group consisted of 15 individuals diagnosed with schizophrenia but with no clinically significant secondary depressive syndrome based on an examination using the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1998). The second patient group was comprised of 15 individuals currently in a major depressive episode and diagnosed with unipolar major depression but without any current or previous psychotic symptoms. As can be seen in Table 1, most individuals in the schizophrenia group were taking atypical antipsychotic medication, and most individuals in the depressed group were taking antidepressants.
Table 1.
Demographic Information by Group
| Schizophrenia (n = 15) |
Depressed (n = 14) |
Control (n = 23) |
|
|---|---|---|---|
| # Female | 7 | 6 | 11 |
| # Caucasian | 12 | 12 | 18 |
| Age M(SD) | 41.47 (5.64) | 43.07 (14.16) | 31.67 (11.39) |
| Medication | |||
| Antipsychotics | 14 | 0 | 0 |
| Antidepressants | 7 | 9 | 0 |
| SSRIs | 6 | 6 | 0 |
| Benzodiazepines | 4 | 0 | 0 |
| Anticonvulsants | 3 | 0 | 0 |
| Other | 1+ | 2++ | 0 |
Note: One participant in the Schizophrenia group was taking a beta-blocker.
One participant in the Depressed group was taking a sleep medication and another participant in the Depressed group was taking a pain medication.
There were 23 participants in the Control group. Control participants were evaluated with SCID-IV and were found to have no lifetime Axis I disorder. Controls also had no lifetime Axis II personality disorders, including no schizophrenia-spectrum personality disorders, based on the Schedule for Schizotypal Personalities (Baron et al., 1981), and no general personality disorder, based on the Inventory of Interpersonal Problems (Pilkonis et al., 1996) and the Personality Disorder Examination (Loranger et al., 1987). Diagnosis agreements were made during case conferences.1
All participants denied any psychoactive drug abuse or alcohol abuse within the past six months and scored in the normal range on a cognitive screen (VIQ equivalent, computed based either on subscales of the WAIS (Wechsler, 1997) or the NAART (Nelson and Willison, 1991). Participants did not report any significant eye problems, and all reported they were able to easily read text on a computer screen much smaller than the text used in the experiment. Participants were excluded if they had a previous history of manic episodes. As can be seen in Table 1, the mean age of the control group was significantly lower than those in both the schizophrenia and depressed groups, F(2,51) = 6.29, p < .01, η2=.2, both ps < .05 from Tukey’s HSD. The groups did not significantly differ on sex or racial composition, both ps > .87.
2.2. Measures of symptoms
2.2.1. Beck Depression Inventory (Beck et al, 1996).
To assess depressive severity in all groups, the Beck Depression Inventory was administered. This 21-item scale asks participants to identify which statement best describes how they have been feeling over the past 2 weeks on a 0-3 scales (e.g., 0 = “I do not feel sad” to 3 = “I am so sad or unhappy that I can’t stand it”).
2.2.2. Anhedonia measures.
Following previous research (e.g., Chapman et al., 1980; Dowd and Barch, 2010) to measure anhedonia in the schizophrenia and control groups, we used two trait anhedonia instruments, the Revised Social Anhedonia Scale (Eckblad et al., 1982) and the Revised Physical Anhedonia Scale (Chapman and Chapman, 1978). Both of these measures are designed to assess trait levels of anhedonia. The Revised Social Anhedonia Scale has 40 true-false items and is designed to measure lack of relationships and lack of pleasure from relationships (e.g., “Having close friends is not as important as many people say”). The Revised Physical Anhedonia Scale has 61 true-false items designed to measure a lack of pleasure gained from physical stimuli, such as food or touch (e.g., “One food tastes as good as another to me”). Two people in the schizophrenia group, five people in the control group, and all participants in the depressed group did not complete the anhedonia measures.
2.3. Emotional valence identification task
As in Siegle et al. (2010), stimuli included 80 positive, 80 negative, and 80 neutral words from the ANEW corpus (Bradley and Lang, 1997). Selected words within each normed affective valence category were balanced for word length, frequency, and arousal, using a computer program designed to create balanced affective word lists (for access, visit http://www.pitt.edu/~gsiegle/wordlist/index.htm). Participants sat approximately 77 cm from the stimuli, which were lowercase letters approximately 1.59 cm high, subtending 1.18° of visual angle, displayed in white on a black computer screen. For each trial (240 trials), participants saw a fixation square for 200 ms. The fixation square was then replaced by a forward mask (a row of Xs) for 2000 ms. The Xs in the forward mask were then replaced by a word from the ANEW list (target stimulus). The target stimulus remained on the screen for 150 ms and then was replaced by a backward mask (a row of Xs), at which time participants were allowed to respond. EEG data were recorded for 8 s after the initial onset of the target stimuli. Thus, the inter-trial interval was 10.35 sec. Participants were told to judge the valence of the stimuli as positive, negative, or neutral as quickly and accurately as they could. Participants’ judgments and reaction times were recorded using button presses on a game-pad capable of reading reaction times with millisecond resolution. To account for differential response latencies to different buttons, the mapping of game-pad buttons to responses was counterbalanced across participants. After the EEG protocol was over, participants rated the valence of the words they had just seen on a 1-7 scale (very negative to very positive).
2.4. Apparatus
EEG data were recorded using methods previously described (Condray et al., 2003; Siegle et al., 2010). In brief, 20-channel EEG data collection was accomplished using a preconfigured Physiometrix cap referenced to nose with forehead as ground. An electrode both above and below the left eye and at the outer canthi of both eyes measured eye movements. Data were recorded via Sensorium amplifiers at a sampling rate of 250 Hz (1 sample/4 ms) using a .02–100 Hz band-pass filter. Using the standard recommended impedance threshold for Sensorium amplifiers, impedances were below 30 kΩ. Data were collected using the EEGSYS software data acquisition platform (Hartwell, 1995).
2.5. Procedure
At a first appointment, participants provided informed consent. All participants received a cognitive screen, and a subset of participants received a brief vision test, which was implemented later in our protocol. At a second appointment, usually within two weeks, participants completed a battery of questionnaire measures and participated in the EEG portion of the study. Testing occurred in a moderately lit room, and an experimenter monitored from an adjacent room. The order of administration of the valence identification task and an unrelated, lexical decision task (not analyzed in this manuscript) was counterbalanced across participants. The tasks were the same as reported in our previous study (Siegle et al., 2010).
2.6. Data retention, cleaning, and reduction
A full description of data retention, cleaning, and reduction procedures is provided in Siegle et al. (2010). In brief, regardless of the judgment of the target, trials were retained, as there is truly no correct response for the emotionality of words. In addition, it was assumed that the process of making a judgment of the word was the same regardless of the actual judgment made.
First, based on previous research, trials with reactions times less than 150 ms were removed from analysis because they were considered spurious responses (Matthews and Southall, 1991). Next, as recommended by Ratcliff (1993), harmonic means of reaction times were calculated as they are a reliably index the central tendency of an individual’s reaction times within a condition. Reaction times of trials were considered outliers if they were outside the Tukey Hinges (1.5 times the interquartile range from the 25th or 75th percentile) on any variable, and were scaled to the closest obtained value below this cutoff plus the difference between this value and the next closest value.
EEG data were processed using a similar procedure as previously reported (Condray et al., 2003; Condray et al., 1999). Artifacts (e.g., blinks, eye movements) with voltage exceeding +/− 150 μV were corrected by applying the Gratton et al. (1983) procedure using the EOG data. Sustained blinks were corrected using linear interpolation. Trials comprised of 50% or more time as a blink were removed. Each trial for each participant was subjected to a wavelet decomposition that yielded a trial X time X frequency matrix for each subject. Power at each frequency during a 200 ms baseline was subtracted for each trial. Average matrices for each valence (positive, negative, and neutral) were computed for each subject of evoked (i.e., nonphase-locked) activity. Mean power in the 35–45 Hz range was extracted for analyses of “gamma” band activity. As detailed below, we characterized the “early” time period as the first two seconds post-stimulus onset and the “late” time period as the last six seconds post-stimulus onset.
2.7. Data analytic strategy
To test whether groups differed in their valence ratings of positive targets, a one-way ANOVA was performed. Pairwise Tukey HSD post hoc tests were then performed. In addition, to test whether groups differed in their reaction times to positive targets, a one-way ANOVA was performed followed by pairwise Tukey HSD post hoc tests.
Primary analyses of the EEG data were conducted using mixed hierarchical linear models (HLM). When analyzing psychophysiological data, multivariate approaches, such as HLM, are robust to incomplete data sets due to artifact rejection and the retention of power by the reduction of error variances through the consideration of the nested nature of the data (i.e., electrodes within subjects, subjects within groups).
Our primary tests examined whether there was an overall difference in gamma band activity between the groups in response to positive stimuli for each electrode across time. To do this, we first conducted an omnibus test to examine whether there was a group by time interaction. After determining that there was such an interaction, our subsequent tests aimed to unpack this interaction. Thus, we examined whether there was a difference in gamma band activity between the groups in response to positive stimuli for each electrode in an “early” time period (i.e., the first two seconds post-stimulus) and in a “late” period (i.e., the last six seconds until the beginning of the next stimulus), as well as differences in activity in the early vs. late periods. We chose these windows through an examination of reaction time data in the schizophrenia group, which was of most interest for this paper. For the schizophrenia group, two seconds was 2 standard deviations above the mean in response times to the positive stimuli (M = 1.48, SD = .26). Thus, we reasoned that “initial processing” occurred prior to the mean response time (i.e., the early time period) and “sustained processing” occurred after such a behavioral response was made (i.e., the late time period).
To test our primary hypotheses, we estimated random intercept models to account for the nested nature of the data, with group X time interactions, one group X time interaction for each time period. A “baseline” period (i.e., one second pre-stimulus onset) was the reference condition for comparing activity in the early and late time periods. Observations were nested within subjects for each of the 20 electrodes. To control type I error for tests of whether there were differences at any specific electrode, a Bonferroni correction was employed in which electrodes significant at p < .05/20, or p < .0025, were considered to represent locations of significant group differences.
We also used a second method to control for type 1 error. Gamma-band EEG is often associated with both cortical and subcortical structures (for a review, see Basar, 2013), which is expected to lead to diffuse surface EEG (Haig et al., 2000; Muller et al., 1999). Thus, tests of group differences considered clusters of significant electrodes, rather than a single electrode, as we were interested in whether there were reliable differences anywhere across the scalp without consideration of specific scalp location. To determine how many electrodes must be significant in order for a group difference to be considered reliable, we subjected a 3-group contrast of baseline-corrected gamma to 1000 Monte-Carlo simulations in which group membership was randomly shuffled. For each simulation, the number of electrodes significant at p < .1 was recorded. Across two replications, we found that 95% of the time, no more than five electrodes were significant atp < .1. Thus, differences between groups were considered reliable (p < .05) if more than five electrodes were significant at p < .1.
Next, we tested whether symptom ratings (BDI, social anhedonia scores, and physical anhedonia scores) were associated with gamma activity. To do this, we examined the correlations between these symptoms and gamma activity within each group. Using the same standard as above, a relationship was considered significant if p < .0025 (i.e., p < .05/20). Finally, given that we previously found differences in gamma band power in response to negative stimuli between the groups (Siegle et al., 2010), we tested whether there were within-group differences in responses to positive vs. negative stimuli. Again, using the same standard as above, an effect was considered reliable if five electrodes or more were significant at p <.1. Because the previous publication reported on group differences in baseline gamma, they were not evaluated here.
3. Results
3.1. Questionnaire measures
As can be seen in Table 2, there was a significant difference between the groups on scores from the Beck Depression Inventory, F(2,51) = 52.02, p < .001, η2= .67. Post hoc analyses revealed that the depressed group had the highest depression scores whereas the control group had the lowest depression scores. The schizophrenia group fell in between the other two groups, all ps < .01. Also, the schizophrenia group had significantly higher scores than the control group on both the Social Anhedonia Scale, t(29) = 3.47, p < .01, d = 1.29, and the Physical Anhedonia Scale, t(29) = 3.44, p < .01, d = 1.28.
Table 2.
Means (SD) of Questionnaire Measures By Group
| Schizophrenia (n = 15) |
Depressed (n = 14) |
Control (n = 23) |
|
|---|---|---|---|
| Social Anhedonia Scale | 15.54 (11.69) | -- | 4.94 (4.81) |
| Physical Anhedonia Scale | 17.0 (9.09) | -- | 8.06 (5.37) |
| Beck Depression Inventory | 13.67 (7.90) | 22.56 (7.54) | 2.52 (2.64) |
| Emotion Ratings of Positive Targets | 5.76 (.55) | 5.39 (.84) | 5.81 (.50) |
| Reaction Times in Seconds in Responses to Positive Targets |
1.48 (.26) | 1.30 (.41) | 1.06 (.33) |
Note: Two people in the schizophrenia group and five people in the control group did not complete the anhedonia scales because these were added to the protocol after these participants completed it.
3.2. Judgment ratings of valence
There was a non-significant group difference in judgments of the valence of the positive stimuli, F(2, 50) = 2.19, p = .12, η2 = .08 (Controls > Schizophrenia > Depressed). There was also a significant group difference in reaction times of judgment ratings, F(2, 50) = 7,34, p < .01, η2 = .23. Posthoc analyses revealed that the control group was significantly faster than the schizophrenia group, p < .01, and non-significantly faster than the depressed group, p = .074. The patient groups did not differ from each other, p = .32.
3.3. Groups differ in gamma activity over time
First, we tested whether groups differed in gamma activity over time in response to positive stimuli. We found that there was, in fact, a reliable difference in gamma activity across groups over time (5 electrodes with p < .1). Thus, we followed up this finding in order to examine how the groups differed in activity across the processing window.
3.4. Schizophrenia group showed greatest activity during the early time period
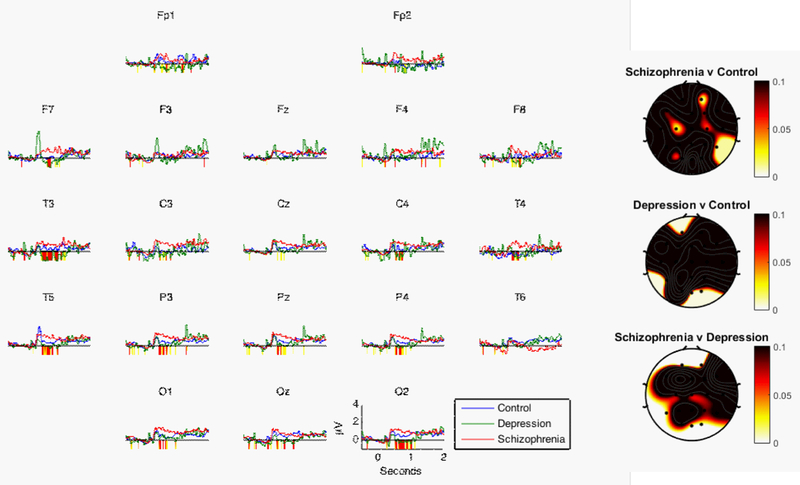
Next, we examined group differences in changes in gamma activity during the early time period (i.e., the first two second post-stimulus onset). As can be seen in Figure 1 and Table 3, the schizophrenia group showed a reliable increase in gamma activity compared to both the control (five electrodes with p < .1) and depressed groups (seven electrodes with p < .1) in the first two seconds of processing positive stimuli. In addition, there were two specific electrode locations that met the threshold for a significant difference between the schizophrenia and depressed groups (Fp2 and O2). In contrast to the results for the schizophrenia group, there was not a reliable difference between the depressed and control groups. This suggests that the schizophrenia group showed the greatest increase in initial processing of positive stimuli.
Figure 1.
Line graphs show mean EEG gamma power (35–45 Hz) over the first two seconds of the processing window by group in response to positive words. Periods of significant differences between the schizophrenia and control groups are highlighted below the x-axis (yellow: p < 0.1; red: p < 0.05). Head plots show p-values for the early period from Table 3.
Table 3.
p-values for Tests of Group Differences in Response to Positive Stimuli
| Baseline vs. Early Time Period post-stimulus onset |
Baseline vs. Late Time Period post-stimulus onset |
Early vs. Late Time Period post-stimulus onset |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrode | SZP vs. Controls |
Depressed vs. Controls |
SZP vs. Depressed |
SZP vs. Controls |
Depressed vs. Controls |
SZP vs. Depressed |
SZP vs. Controls |
Depressed vs. Controls |
SZP vs. Depressed |
| Fz | .28 | .72 | .52 | .78 | .07 | .06 | .05 | .07 | <.001 |
| Cz | .13 | .76 | .09 | .96 | .19 | .25 | .03 | .03 | <.001 |
| Pz | .31 | .68 | .20 | .89 | .05 | .06 | .09 | .001 | <.001 |
| Oz | .99 | .01 | .01 | .13 | .88 | .13 | .05 | <.001 | <.001 |
| Fp1 | .57 | .02 | .01 | .41 | .88 | .54 | .05 | .001 | <.001 |
| F3 | .23 | .39 | .75 | .88 | .08 | .08 | .05 | .29 | .01 |
| F7 | .10 | .58 | .32 | .88 | .18 | .17 | .01 | .34 | .001 |
| C3 | .02 | .52 | .11 | .34 | .06 | .41 | .02 | .14 | <.001 |
| P3 | .17 | .99 | .21 | .51 | .04 | .01 | .003 | .01 | <.001 |
| T3 | .32 | .44 | .11 | .09 | .70 | .06 | <.001 | .09 | <.001 |
| T5 | .25 | .13 | .02 | .59 | .37 | .19 | .02 | <.001 | <.001 |
| O1 | .19 | .68 | .12 | .62 | .31 | .17 | .01 | .05 | <.001 |
| Fp2 | .01 | .49 | .002 | .85 | .80 | .69 | <.001 | .48 | <.001 |
| F4 | .08 | .53 | .29 | .87 | .005 | .02 | .01 | .01 | <.001 |
| F8 | .37 | .42 | .93 | .44 | .001 | <.001 | .02 | .002 | <.001 |
| C4 | .06 | .81 | .13 | .49 | <.001 | .02 | .05 | <.001 | <.001 |
| P4 | .42 | .30 | .09 | .76 | .04 | .03 | .11 | <.001 | <.001 |
| T4 | .14 | .85 | .25 | .52 | .004 | .002 | .003 | <.001 | <.001 |
| T6 | .01 | .34 | .12 | .48 | .38 | .15 | .002 | .01 | .68 |
| O2 | .40 | .004 | <.001 | .54 | .30 | .13 | .04 | <.001 | <.001 |
| # of electrodes with p < .1 |
5 | 3 | 7 | 1 | 10 | 10 | 19 | 16 | 19 |
Note: SZP = schizophrenia; Bold = p values significant at p < .0025, which represents a significant result at that specific electrode at p < .05 after accounting for multiple tests across electrodes; Italics = p values significant at p < .1. Monte Carlo simulations suggested that for these data, 5 electrodes significant at p < .1 were needed to suggest there were reliable differences between groups at a level of p < .05.
3.5. Depressed group showed the greatest activity during the late time period
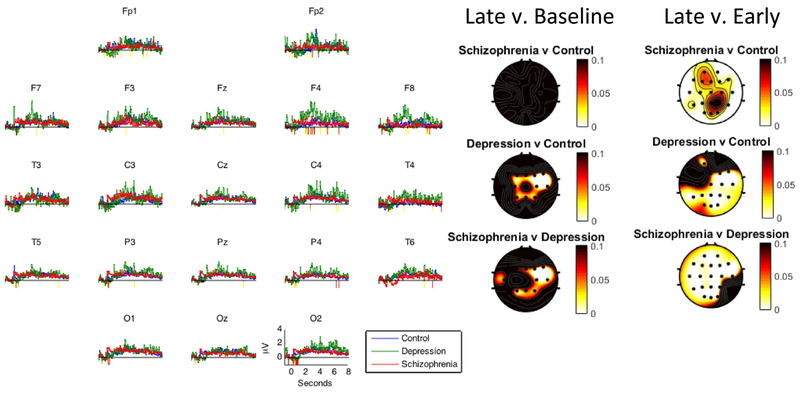
We then examined group differences in changes in gamma activity during the late time period (i.e., the last six second post-stimulus onset). As can be seen in Figure 2 and Table 3, the depressed group showed a reliable increase in gamma activity in the late time period compared to both the control group (10 electrodes with p < .1) and schizophrenia group (10 electrodes with p < .1). Also, there were two specific electrode locations that met the threshold for a significant difference between the depressed and controls groups (F8 and C4) and between the depressed and schizophrenia groups (F8 and T4). In contrast to the results for the depressed group, there was not a reliable difference between the schizophrenia and control groups. This suggests that the depressed group showed the greatest increase in elaborative processing of positive stimuli over time.
Figure 2.
Line graphs show mean EEG gamma power (35–45 Hz) over the entire processing window (i.e., eight seconds) by group in response to positive words. Periods of significant differences between the schizophrenia and control groups are highlighted below the x-axis (yellow: p < 0.1; red: p < 0.05). Leftmost head plots show p-values for the late minus the baseline period from Table 3; Rightmost head plots show p-values for the late minutes the early period from Table 3.
3.6. Schizophrenia group showed greatest decrease and depressed group showed the greatest increase in activity in the early vs. the late period
We were also interested in whether there were significant differences between activity associated with initial processing and sustained processing. Thus, we examined group differences in changes in gamma activity from the early to the late time period. As can be seen in Figure 2 and Table 3, the schizophrenia group showed a reliable decrease in gamma activity from the early to the late time period compared to both the control (19 electrodes with p < .1) and depressed groups (19 electrodes with p < .1) when processing positive stimuli. In addition, the depressed group showed a reliable increase in gamma activity from the early to the late time period compared to the control group (16 electrodes with p < .1). Also, there were a number of specific electrode locations that meet the threshold for a significant difference between groups (Schizophrenia vs. Controls = 3 electrodes; Schizophrenia vs. Depressed = 18; Depressed vs. Controls = 9). This suggests that the schizophrenia group showed the greatest decrease, while depressed group showed the greatest increase, in the early vs. late period.
3.7. No evidence of an impact of age or behavioral reaction time
Because the groups differed in age, we tested for potential age effects by creating a “better-age-matched” subset of participants by eliminating the 10 youngest controls, the 2 oldest participants with schizophrenia, and the oldest participant with depression. This yielded 13 subjects per group. The mean ages of the new groups did not significantly differ, F(2,38)=.45, p=.64). We then re-ran all of the analyses using these new groups. Overall, the pattern of results was identical as the original data. For example, the schizophrenia group showed a reliable increase in gamma activity compared to the control group during the early period but showed no difference in gamma activity compared to this group during the late period. Thus, it is unlikely that results based on the full sample were due to age.
Because the groups differed in reaction times (RT) to positive stimuli, we tested for potential effects by creating a “better-RT-matched” subset of participants by eliminating the 10 fastest controls, the 2 slowest participants with schizophrenia, and the slowest participant with depression. This yielded 13 subjects per group. . The mean RT of the new groups did not significantly differ, F(2,38) = 2.04, p = .15. We then re-ran all of the analyses using these new groups. Overall, the pattern of results was identical as the original data. For example, the schizophrenia group showed a reliable increase in gamma activity compared to the control group during the early period but showed no difference in gamma activity compared to this group during the late period. Thus, it is unlikely that results based on the full sample were due to behavioral reaction times to positive stimuli.
3.8. Self-reported depression and social anhedonia are associated with decreased elaborative processing in the schizophrenia group
We then tested whether self-reported depression or anhedonia was related to changes in gamma activity in the early time period or the late time period in the patient groups. We found that in the schizophrenia group, increases in BDI and increases in social anhedonia scores were associated with decreases in gamma activity across time [BDI: r(300)= −.28, p < .001; Social anhedonia: r(260)= −.26, p < .001]. This same pattern was found for physical anhedonia, although the relationship was not significant once corrected for multiple comparisons, r(260)= −.13, p = .032. In contrast to the schizophrenia group, increases in BDI scores were associated with increases in gamma activity across time for the depressed group, but this relationship was not significant once corrected for multiple comparisons, r(260)= .17, p = .008.
3.9. Responses to positive vs. negative words differ between groups
Last, we tested whether the groups had differing responses to positive vs. negative stimuli collapsing across time. Examining within-group effects, as can be seen in Table 4, we found that only the depressed group showed reliably increased responses to negative compared to positive stimuli. When examining between-group effects, we found that compared to the control group, the depressed group had a reliably increased response to negative stimuli compared to positive (9 electrodes with p < .1) whereas the schizophrenia group had a reliably increased response to positive stimuli compared to negative stimuli (9 electrodes with p < .1). Similarly, compared to the depressed group, the schizophrenia group had a reliably increased response to positive stimuli compared to negative stimuli (18 electrodes with p < .1).
Table 4.
t-tests of differences in gamma power in response to positive versus negative words across electrodes for the entire processing window (8 sec post-stimulus onset).
| Electrode | Control |
Depressed |
Schizophrenia |
||||||
|---|---|---|---|---|---|---|---|---|---|
| t(22) | p | d | t(14) | p | d | t(14) | p | d | |
| Fz | 0.55 | .59 | 0.11 | −0.77 | .46 | −0.20 | 0.49 | .63 | 0.13 |
| Cz | −0.01 | .99 | 0.00 | −1.55 | .14 | −0.40 | −0.03 | .98 | −0.01 |
| Pz | −0.66 | .51 | −0.14 | −1.52 | .15 | −0.39 | 0.32 | .76 | 0.08 |
| Oz | −0.99 | .33 | −0.21 | −2.19 | .05 | −0.57 | 0.89 | .39 | 0.23 |
| Fp1 | 0.44 | .67 | 0.09 | −17 | .26 | −0.30 | 0.88 | .39 | 0.23 |
| F3 | −0.06 | .95 | −0.01 | −0.85 | .41 | −0.22 | −1.15 | .27 | −0.30 |
| F7 | −1.25 | .22 | −0.26 | −1.92 | .08 | −0.50 | 0.25 | .81 | 0.06 |
| C3 | −1.01 | .32 | −0.21 | −2.37 | .03 | −0.61 | −0.76 | .46 | −0.20 |
| P3 | −0.64 | .53 | −0.13 | −1.43 | .18 | −0.37 | 0.26 | .79 | 0.07 |
| T3 | −0.77 | .45 | −0.16 | −1.73 | .11 | −0.45 | −0.43 | .68 | −0.11 |
| T5 | −0.03 | .98 | −0.01 | −2.59 | .02 | −0.67 | 0.36 | .72 | 0.09 |
| O1 | −0.10 | .92 | −0.02 | −1.14 | .27 | −0.29 | 1.22 | .24 | 0.32 |
| Fp2 | 0.72 | .48 | 0.15 | −0.27 | .79 | −0.07 | 1.98 | .07 | 0.51 |
| F4 | −0.54 | .59 | −0.11 | −0.36 | .72 | −0.09 | 0.15 | .89 | 0.04 |
| F8 | −0.15 | .88 | −0.03 | −1.53 | .15 | −0.40 | 0.82 | .43 | 0.21 |
| C4 | −0.66 | .52 | −0.14 | −2.02 | .06 | −0.52 | 0.66 | .52 | 0.17 |
| P4 | −1.45 | .16 | −0.30 | −2.22 | .04 | −0.57 | 0.85 | .41 | 0.22 |
| T4 | −1.10 | .28 | −0.23 | −1.27 | .22 | −0.33 | −0.15 | .89 | −0.04 |
| T6 | −0.70 | .49 | −0.15 | −0.77 | .45 | −0.20 | 1.60 | .13 | 0.41 |
| O2 | −0.07 | .95 | −0.01 | −0.79 | .45 | −0.20 | 0.55 | .59 | 0.14 |
| # of electrodes with p < .1 |
0 | 6 | 1 | ||||||
Note: d represent Cohen’s d, calculated as the t value divided by the square root of n. Bold = values significant at p <.1.
4. Discussion
The current findings highlight nuances with respect to gamma band activity in response to positive stimuli, theorized to index elaborative processing, in a group of individuals with schizophrenia and a group with depression compared to healthy individuals. In addition, they highlight the importance of depressive symptomology and social anhedonia when considering emotional abnormalities in schizophrenia.
The finding of the greatest activity for the schizophrenia group during the early time period suggests that the emotional information might be especially attention-grabbing, potentially due to a novelty effect. That is, it is possible that because individuals with schizophrenia do not typically attend to positive emotional stimuli, the exposure to such stimuli while instructed to attend to it, was novel experientially. This finding is consistent with previous findings of increased early physiological responses to emotional stimuli in schizophrenia (Duval et al., 2016; Holt et al., 2006; Kosaka et al., 2002; Kring and Neale, 1996).
At the same time, the finding of the greatest activity during the early time period coupled with a reliable decrease in activity from the early to the late time period for the schizophrenia group suggests that individuals with schizophrenia engage in less efficient processing of positive stimuli. This finding could thus be consistent with previous literature of intact or at least functionally compensated “in-the-moment” or consummatory pleasure both in the laboratory and in daily life (Kring and Moran, 2008). For example, in response to a variety of types of pleasant stimuli, Burbridge and Barch (2007) found that individuals with schizophrenia reported similar experiences of pleasant affect (but not similar arousal levels) as a control group. In addition, using experience sampling, Gard and colleagues (2007) reported that people with schizophrenia have similar experiences of positive affect when engaged in goal or non-goal directed activity. At the same time, the current finding of a reliable decrease in sustained processing in the schizophrenia group compared to the other groups is consistent with previous reports of decreased trait positive affect. For example, schizophrenia is consistently associated with reports of increased trait anhedonia compared to control participants (e.g., Cohen et al., 2005; Horan et al., 2008; Martin et al., 2013). Thus, it is possible that because people with schizophrenia show a decreased in sustained elaborative processing of pleasant stimuli compared healthy individuals their feelings of positive affect elicited when exposed to a stimulus are not encoded and consolidated in memory in the same way. Given that elaborative processing is associated with enhanced memory recall (Anderson, 2005; Anderson and Reder, 1979), a lack of in-depth processing may be related to the difficulty people with schizophrenia have when trying to recall such feelings, leading to reports of decreased trait positive affect.
The current finding of decreased sustained gamma band activity in schizophrenia is also consistent with research that has investigated evoked gamma band activity in this group using non-emotional stimuli. The majority of studies utilized auditory stimuli and have reported decreased gamma band activity in schizophrenia. For example, auditory steady-state studies generally reported reduced gamma activity in schizophrenia in response to a tone (e.g., Light et al., 2006; Roach and Mathalon, 2008; Spencer et al., 2008). However, the little work that has used visual stimuli is more mixed with some reporting no differences (e.g., Krishnan et al., 2005) and others reporting reduced activity in schizophrenia (e.g., Riecansky et al., 2010). Hence, it is possible that stimulus modality impacts evoked gamma band responses. Future research could utilize both auditory and visual emotional stimuli in the same study to examine possible differences in elaborative processing in schizophrenia.
Of note, although the groups differed in initial reactivity (and sustained processing from the early to the late period), the schizophrenia group did not differ from the control group in gamma activity during the late period. Notably, no group differences during the late period is not inconsistent with a decreased ability to savor positive emotions. That is, people with schizophrenia may engage in ineffective elaboration of positive stimuli in that they are not embellishing the stimuli in a way that allows for effective encoding, consolidation, and subsequent recall of these positive feelings. Overall, these results suggest that schizophrenia is associated with decreased time spent engaging in elaborative processing of positive stimuli, which could be related to decreased trait positive affect.
EEG gamma has been associated with the inhibitory neurotransmitter, GABA, and the excitatory neurotransmitter, glutamate, including their receptors (e.g., NMDA-type glutamate receptor) (Komek et al., 2012). It has been suggested that some gamma oscillations originate within networks of inhibitory GABAergic interneurons and that this process may be driven by glutamate receptor activation (for a review, see Basar, 2013). Thus, dysfunction of these neurotransmitters, as is seen in schizophrenia (Wassef et al., 2003), could lead to abnormal gamma activity. In addition, gamma has been strongly associated with dopaminergic function. For example, both reward and amphetamine challenge change EEG gamma frequency responses in animals (Berke, 2009) particularly in the ventral tegmental area (VTA), which produces dopamine (Kalenscher et al., 2010; van der Meer et al., 2010). In humans, reward is associated with the dopaminergic activity, including gamma activity involving the nucleus accumbens (Cohen et al., 2009). Furthermore, interventions for schizophrenia directed at dopamine-modulated GABA receptor function (Erickson et al., 2000) also alter frontal gamma power (Lewis et al., 2008). Given that the majority of people with schizophrenia are prescribed medications that serve as dopamine antagonists, it is possible that reductions in gamma band activity could be due to medication effects. In the current study, 93% of individuals in the schizophrenia group were treated with antipsychotic medication acting as dopamine antagonists and thus we were not able to examine medication effects. However, the schizophrenia group showed increased gamma activity during the early time period, suggesting the early vs. late changes are not attributable to medication. Nevertheless, future research could utilize at-risk, unmedicated (e.g., first-episode patients) and medicated samples in the same study to examine possible medication effects in gamma band activity in response to emotional stimuli. In addition, future research could test whether chlorpromazine equivalent doses are associated with gamma activity as they were unavailable in the current study.
In contrast to the schizophrenia group, the depression group did not differ from controls in initial gamma activity but did show a reliable sustained increase. This is consistent with findings from a recent meta-analysis of functional brain imaging studies (Groenewold et al., 2013) that found depression was associated with increased activity in the orbitofrontal cortex in response to pleasant stimuli as well as increased activity in a number of other regions (e.g., parahippocampal gyrus, amygdala) in response to unpleasant stimuli. Given that self-report and behavioral work consistently reflects poor memory for positive information (Blaney, 1986; MacLeod and Mathews, 1991; Matt et al., 1992) and decreased positive elaboration in depression (Horner et al., 2014), it is possible the individuals with depression are either interpreting the positive stimuli as negative or engaging in over-regulation of the positive stimuli. At the same time, we found that the depressed group had greater evoked gamma band activity to negative stimuli compared to positive stimuli whereas the gamma activity between positive and negative stimuli did not differ for the other two groups. For the depressed group, this finding is consistent with the “negativity bias” in that negative information is processed to a greater extent than positive information (e.g., Baumeister et al., 2001).
In the current study, self-reported depressive symptoms in the schizophrenia group was associated with initial and sustained decreased gamma reactivity. This is consistent with findings of decreased state positive affect in those with depressive symptoms (e.g., Dunn et al., 2004; Sloan et al., 2001), as well as multiple cognitive impairments. This finding highlights the importance of the consideration of depressive symptomology as a potential moderator of emotional abnormalities in schizophrenia. Thus, future research on emotional abnormalities in schizophrenia may benefit for the standard assessment and analysis of depressive symptoms. In addition, self-reported social anhedonia in the schizophrenia group was associated with decreased gamma reactivity over the entire processing window. This is consistent with reports of a negative relationship between anhedonia and savoring in schizophrenia-prone individuals (Applegate et al., 2009). Although these data were not available for the depressed group (because we did not collect self-report measures of anhedonia from the depressed group), we hypothesize a similar effect would be found in this group given the critical role anhedonia plays in treatment outcomes. For example, anhedonia in adolescents and adults uniquely predicts longer time to remission of depressive symptoms and treatment non-response (McMakin et al., 2012; Vrieze et al., 2014). Thus, future research could include a measure of self-reported anhedonia to test for associations as was done for the schizophrenia group here. In addition, future research could test for associations of clinician-rated symptoms and gamma band activity to investigate whether differential associations are observed.
Overall, these results indicate that initial processing of positive stimuli is intact in schizophrenia; however, these individuals have difficulty sustaining such processing and this abnormality is associated with self-reported symptoms of low positive affect. Thus, when targeting negative symptoms, it may be more advantageous to foster savoring of positive experiences (Favrod et al., 2015; Nguyen et al., 2016), rather than targeting negative cognitions, to capitalize on potentially intact automatic processes.
Acknowledgements
We would like to thank Matcheri Keshavan, MD, for his involvement and support of this work at Western Psychiatric Institute and Clinic.
Role of the Funding Source
This work was supported by MH50631, NARSAD, MH64159, the Veteran’s Research Foundation, MH30915, MH58356, and MH60473. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Although no participants in the study met criteria for a substance use disorder, we did not confirm an absence of use with urine toxicology screens.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None.
7. References
- Anderson JR, 2005. Cognitive Psychology and its Implications, 6th ed. Worth, New York. [Google Scholar]
- Anderson JR, Reder LM, 1979. An elaborative prcessing explanation of depth of processing, Levels of procesisng in human memory. Lawrence Erlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Applegate E, El-Deredy W, Bentall RP, 2009. Reward responsiveness in psychosis-prone groups: Hypomania and negative schizotypy. Pers. Individ. Dif 47, 452–456. [Google Scholar]
- Baron M, Asnis L, Gruen R, 1981. The Schedule for Schizotypal Personalities (SSP): A Diagnostic Interview for Schizotypal Features. Psych. Res, 4, 213–228. [DOI] [PubMed] [Google Scholar]
- Basar E, 2013. A review of gamma oscillations in healthy subjects and in cognitive impairment. Int. J. Psychophysiol 90, 99–117. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD, 2001. Bad is stronger than good. Rev. Gen. Psychol 5, 323–370. [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Berke JD, 2009. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci 30, 848–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand O, Tallon-Baudry C, 2000. Oscillatory gamma activity in humans: a possible role for object representation. Int. J. Psychophysiol 38, 211–223. [DOI] [PubMed] [Google Scholar]
- Blaney PH, 1986. Affect and memory: a review. Psychol. Bull 99, 229–246. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ, 2001. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion 1, 276–298. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, 1997. Affective Norms for English Words ANEW Technical Manual and Affective Ratings The Center for Reserach in Psychophysiology, University of Florida, Gainsville, FL. [Google Scholar]
- Burbridge JA, Barch DM, 2007. Anhedonia and the experience of emotion in individuals with schizophrenia. J. Abnorm. Psychol 116, 30–42. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, 1978. Revised Scale for Physical Anhedonia, University of Wisconsin. [Google Scholar]
- Chapman LJ, Edell WS, Chapman JP, 1980. Physical anhedonia, perceptual aberration, and psychosis proneness. Schizophr. Bull 6, 639–653. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Dinzeo TJ, Nienow TM, Smith DA, Singer B, Docherty NM, 2005. Diminished emotionality and social functioning in schizophrenia. J. Nerv. Ment. Dis 193, 796–802. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS, 2010. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull 36, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE, 2009. Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J. Cogn. Neurosci 21, 875–889. [DOI] [PubMed] [Google Scholar]
- Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR, 2003. Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biol. Psychiatry 54, 1134–1148. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, Cohen JD, van Kammen DP, Kasparek A, 1999. Modulation of language processing in schizophrenia: effects of context and haloperidol on the event-related potential. Biol. Psychiatry 45, 1336–1355. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS, 1972. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior 11, 671–684. [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK, 2003. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport 14, 683–686. [DOI] [PubMed] [Google Scholar]
- Dowd EC, Barch DM, 2010. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol. Psychiatry 67, 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD, Cusack R, Ogilvie AD, 2004. Categorical and dimensional reports of experienced affect to emotion-inducing pictures in depression. J. Abnorm. Psychol 113, 654–660. [DOI] [PubMed] [Google Scholar]
- Duval CZ, Goumon Y, Kemmel V, Kornmeier J, Dufour A, Andlauer O, Vidailhet P, Poisbeau P, Salvat E, Muller A, Mensah-Nyagan AG, Schmidt-Mutter C, Giersch A, 2016. Neurophysiological responses to unpleasant stimuli (acute electrical stimulations and emotional pictures) are increased in patients with schizophrenia. Sci. Rep 6, 22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M, 1982. The Revised Social Anhedonia Scale. [Google Scholar]
- Engel AK, Singer W, 2001. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 5, 16–25. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Sesack SR, Lewis DA, 2000. Dopamine innervation of monkey entorhinal cortex: postsynaptic targets of tyrosine hydroxylase-immunoreactive terminals. Synapse 36, 47–56. [DOI] [PubMed] [Google Scholar]
- Favrod J, Nguyen A, Fankhauser C, Ismailaj A, Hasler JD, Ringuet A, Rexhaj S, Bonsack C, 2015. Positive Emotions Program for Schizophrenia (PEPS): a pilot intervention to reduce anhedonia and apathy. BMC Psychiatry 15, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 1998. Structured Clinical Interview for DSM-IV Axis I Disorders New York State Psychiatric Institute, New York. [Google Scholar]
- Fries P, 2009. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci 32, 209–224. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D, 2004. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin. Neurophysiol 115, 1863–1874. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF, 2007. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res 93, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT, 2012. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry 69, 121–127. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol 55, 468–484. [DOI] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG, 2013. Emotional valence modulates brain functional abnormalities in depression: evidence from a metaanalysis of fMRI studies. Neurosci. Biobehav. Rev 37, 152–163. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, Wright JJ, Meares RA, Bahramali H, 2000. Synchronous cortical gamma-band activity in task-relevant cognition. Neuroreport 11, 669–675. [DOI] [PubMed] [Google Scholar]
- Hartwell JW, 1995. EEGSYS Version 5.7 User’s Guide. Friends Medical Science Research Center, Inc, Balitmore, MD. [Google Scholar]
- Headley DB, Pare D, 2013. In sync: gamma oscillations and emotional memory. Front. Behav. Neurosci 7, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Fox AS, Wing EK, McQuisition KM, Vack NJ, Davidson RJ, 2015. The Neurodynamics of Affect in the Laboratory Predicts Persistence of Real-World Emotional Responses. J. Neurosci 35, 10503–10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener ES, 2008. Emotional memory in schizophrenia. Schizophr. Bull 34, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S, 2006. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr. Res 82, 153–162. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF, 2008. Affective traits in schizophrenia and schizotypy. Schizophr. Bull 34, 856–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MS, Siegle GJ, Schwartz RM, Price RB, Haggerty AE, Collier A, Friedman ES, 2014. C’mon get happy: reduced magnitude and duration of response during a positive-affect induction in depression. Depress. Anxiety 31, 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DP, Penn DL, Fredrickson BL, Kring AM, Meyer PS, Catalino LI, Brantley M, 2011. A pilot study of loving-kindness meditation for the negative symptoms of schizophrenia. Schizophr. Res 129, 137–140. [DOI] [PubMed] [Google Scholar]
- Johnson EL, Knight RT, 2015. Intracranial recordings and human memory. Curr. Opin. Neurobiol 31, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH, 2007. Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol 116, 80–85. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH, 2007. Biased processing of emotional information in girls at risk for depression. J. Abnorm. Psychol 116, 135–143. [DOI] [PubMed] [Google Scholar]
- Kalenscher T, Lansink CS, Lankelma JV, Pennartz CM, 2010. Reward-associated gamma oscillations in ventral striatum are regionally differentiated and modulate local firing activity. J. Neurophysiol 103, 1658–1672. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Martin EA, 2006. Emotional processing in schizophrenia. Cogn. Neuropsychiatry 11, 250–271. [DOI] [PubMed] [Google Scholar]
- Komek K, Bard Ermentrout G, Walker CP, Cho RY, 2012. Dopamine and gamma band synchrony in schizophrenia--insights from computational and empirical studies. Eur. J. Neurosci 36, 2146–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, Takahashi T, Sadato N, Itoh H, Yonekura Y, Wada Y, 2002. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr. Res 57, 87–95. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK, 2008. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull 34, 819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Neale JM, 1996. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J. Abnorm. Psychol 105, 249–257. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF, 2005. Steady state visual evoked potential abnormalities in schizophrenia. Clin. Neurophysiol 116, 614–624. [DOI] [PubMed] [Google Scholar]
- Kucewicz MT, Cimbalnik J, Matsumoto JY, Brinkmann BH, Bower MR, Vasoli V, Sulc V, Meyer F, Marsh WR, Stead SM, Worrell GA, 2014. High frequency oscillations are associated with cognitive processing in human recognition memory. Brain 137, 2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E, 2003. “Gamma (40 Hz) phase synchronicity” and symptom dimensions in schizophrenia. Cogn. Neuropsychiatry 8, 57–71. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D, 2008. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am. J. Psychiatry 165, 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL, 2006. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol. Psychiatry 60, 1231–1240. [DOI] [PubMed] [Google Scholar]
- Llerena K, Strauss GP, Cohen AS, 2012. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr. Res 142, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenger AW, Susman VL, Oldham JM, Russakoff LM, 1987. The Personality Disorder Examination: A preliminary report. Journal of Pers. Disorders, 1,1–13. [Google Scholar]
- MacLeod C, Mathews A, 1991. Biased cognitive operations in anxiety: accessibility of information or assignment of processing priorities? Behav. Res. Ther 29, 599–610. [DOI] [PubMed] [Google Scholar]
- Martin EA, Becker TM, Cicero DC, Kerns JG, 2013. Examination of affective and cognitive interference in schizophrenia and relation to symptoms. J. of Abnormal Psych 122, 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Vazquez C, Campbell WK, 1992. Mood-congruent recall of affective toned stimuli: A meta-analytic review. Clin. Psychol. Rev 12, 227–255. [Google Scholar]
- Matthews G, Southall A, 1991. Depresison and the processing of emotional stimuli: A study of semantic priming. Cognit. Ther. Res 15, 282–302. [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR, Ryan ND, Birmaher B, Shamseddeen W, Mayes T, Kennard B, Spirito A, Keller M, Lynch FL, Dickerson JF, Brent DA, 2012. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J. Am. Acad. Child Adolesc. Psychiatry 51, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Siegle GJ, Shirk SR, 2011. Positive Affect Stimulation and Sustainment (PASS) Module for Depressed Mood: A preliminary investigation of treatment-related effects. Cognit. Ther. Res 35, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MM, Keil A, Gruber T, Elbert T, 1999. Processing of affective pictures modulates right-hemispheric gamma band EEG activity. Clin. Neurophysiol 110, 1913–1920. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA, deVries MW, 2000. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr. Bull 26, 847–854. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J, 1991. The National Adult Reading Test. Nfer-Nelson Publishing, Berkshire, England. [Google Scholar]
- Nguyen A, Frobert L, McCluskey I, Golay P, Bonsack C, Favrod J, 2016. Development of the Positive Emotions Program for Schizophrenia: An Intervention to Improve Pleasure and Motivation in Schizophrenia. Front Psychiatry 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M, Kwapil T, Delespaul P, Myin-Germeys I, 2009. Momentary assessment research in psychosis. Psychol. Assess 21, 498–505. [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Yookyung K, Proietti JM, Barkham M, 1996. Scales for personality disorders developed from the Inventory of Interpersonal Problems. J. of Pers. Dis, 10, 355–369. [Google Scholar]
- Ratcliff R, 1993. Methods for dealing with reaction time outliers. Psychol. Bull 114, 510–532. [DOI] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE, 2008. High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin. Neurophysiol 119, 116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecansky I, Kasparek T, Rehulova J, Katina S, Prikryl R, 2010. Aberrant EEG responses to gamma-frequency visual stimulation in schizophrenia. Schizophr. Res 124, 101–109. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH, 2008. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull 34, 907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D, Kautz J, Hauck M, Zimmermann R, Engel AK, 2011. Emotional facial expressions modulate pain-induced beta and gamma oscillations in sensorimotor cortex. J. Neurosci 31, 14542–14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Condray R, Thase ME, Keshavan M, Steinhauer SR, 2010. Sustained gamma-band EEG following negative words in depression and schizophrenia. Int. J. Psychophysiol 75, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL, 2001. Diminished response to pleasant stimuli by depressed women. J. Abnorm. Psychol 110, 488–493. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW, 2008. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol. Psychiatry 64, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM, 2012. A new perspective on anhedonia in schizophrenia. American Journal of Psychiatry 169, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MA, Kalenscher T, Lansink CS, Pennartz CM, Berke JD, Redish AD, 2010. Integrating early results on ventral striatal gamma oscillations in the rat. Front. Neurosci 4, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, Hompes T, de Boer P, Schmidt M, Claes S, 2014. Dimensions in major depressive disorder and their relevance for treatment outcome. J. Affect. Disord 155, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD, 2003. GABA and schizophrenia: a review of basic science and clinical studies. J. Clin. Psychopharmacol 23, 601–640. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler Adult Intelligence Scale, 3rd ed. Psychological Corportation, San Antonio, TX. [Google Scholar]
- Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J, 2014. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One 9, e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]