Abstract
Introduction
Although diabetes mellitus (DM) is often discussed as a risk factor for inflatable penile prosthesis (IPP) infection, the link between DM diagnosis and IPP infection remains controversial. High-quality population-based data linking DM to an increased risk of IPP infection have not been published.
Aim
To evaluate the association of DM with IPP infection in a large public New York state database.
Methods
The New York Statewide Planning and Research Cooperative System (SPARCS) database was queried for men who underwent initial IPP insertion from 1995–2014. Diabetic patients were identified using ICD-9-CM codes. Patients presenting for first operation with diagnosis or Current Procedural Terminology codes suggestive of prior IPP surgery were excluded. Chi-squared analyses were performed to compare infection rates in diabetics and non-diabetics within the pre- and postantibiotic impregnated eras. Multivariate Cox proportional hazards models were constructed to evaluate whether or not DM was independently associated with IPP infection in the time periods before (1995–2003) and after (2004–2014) the widespread availability of antibiotic impregnated penile prostheses.
Main Outcome Measure
Time to prosthesis infection was measured.
Results
14,969 patients underwent initial IPP insertion during the study period. The overall infection rate was 343/14,969 (2.3%). Infections occurred at a median 3.9 months after implant (interquartile ratio: 1.0–25.0 months). Infectious complications were experienced by 3% (133/4,478) of diabetic patients and 2% (210/10,491) of non-diabetic patients (P < .001). Diabetes was associated with a significantly increased IPP infection risk on multivariable analysis controlling for age, race, comorbidities, insurance status, annual surgeon volume, and era of implantation (Hazard Ratio: 1.32, 95% CI: 1.05–1.66, P = .016).
Conclusion
Our analysis supports the notion that DM is a risk factor for IPP infection. This has important implications for patient selection and counseling, and raises the question of whether this increased risk can be mitigated by optimization of glycemic control before surgery.
Lipsky MJ, Onyeji I, Golan R, et al. Diabetes Is a Risk Factor for Inflatable Penile Prosthesis Infection: Analysis of a Large Statewide Database. Sex Med 2019;7:35–40.
Key Words: Erectile Dysfunction, IPP, Penile Implant, Diabetes, Infection
Introduction
Diabetes is a risk factor for the development of erectile dysfunction (ED), with an estimated relative risk of 1.83 compared with non-diabetics.1 The prevalence of ED among diabetic men is estimated to be 50%.2 Diabetes may lead to ED through multiple potential pathways including neuropathy, endothelial dysfunction, cavernosal smooth muscle changes, and connective tissue changes that impair tunical elasticity and result in veno-occlusive dysfunction. The risk of ED in diabetic men increases in those with insulin-dependent diabetes or diabetes-associated comorbidities such as nephropathy, retinopathy, neuropathy, and cardiovascular disease.3
Penile prosthesis surgery is a successful treatment option associated with high satisfaction rates among men with ED.4 In fact, diabetic men were found to have double the odds of undergoing penile implant surgery compared with non-diabetic men with ED using a large nationwide database.5 Diabetic men theoretically pose unique challenges to surgeons because they are at risk for delayed wound healing and generally increased risks of postoperative infection,6 which are particularly concerning risks when implanting a foreign device. In fact, diabetes has been identified as a risk factor for implant infection when analyzing cases of non-urologic surgery.7, 8
The current rate of inflatable penile prosthesis (IPP) infections is 0.5–2%, notably lower than the reported 2–5% rate before the introduction of antibiotic-coated implants.9, 10 A number of risk factors for the development of IPP infection have been identified. These include implant revision surgery compared with primary implantation,11 chronic steroid use, and spinal cord injury as the cause of ED.12 Although the association between diabetes (or poor glycemic control) and IPP infection has been posited, the results of multiple prior studies have been mixed.11, 12, 13, 14, 15, 16, 17 We sought to analyze the impact of diabetes on the infection risk in patients presenting for an initial IPP placement using a large statewide population-based dataset.
Materials and Methods
Data Extraction
Our study was determined to be exempt from formal review by our institutional review board. We queried the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify patients in New York state who underwent initial IPP implantation between 1996 and 2014. The SPARCS database is a de-identified comprehensive, all-payer administrative database that collects patient, physician, and hospital data from each inpatient hospital admission, emergency room visit, and hospital-based ambulatory surgery visit in New York state. Patients are assigned individualized identifier codes upon their first encounter at a SPARCS facility, which allows geographic and temporal patient tracking in the database. Individual physicians may be identified by license number, which is recorded for each clinical encounter. Hospitals are identified by facility name and county. Patient information collected includes demographic information, insurance status, comorbid conditions (coded for each visit) as well as procedures for each visit with associated date. In addition, location and attending physician performing each procedure are identifiable based on hospital codes as well as medical license numbers, respectively.
Patient Identification
We queried the SPARCS database for all patients who underwent initial IPP implantation between 1996 and 2014 in New York State. This included procedures performed both in inpatient facilities and in ambulatory settings. Because procedures in SPARCS are coded with either International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes or Current Procedural Terminology (CPT) codes, we identified patients with ICD-9-CM code 6497 (Insertion or replacement of IPP) as well as CPT codes 54401 (Insertion of penile prosthesis; inflatable [self-contained]) and 54405 (Insertion of multi-component, inflatable penile prosthesis, including placement of pump, cylinders, and reservoir). To ensure that we only included primary implants, we excluded patients with diagnoses or procedural codes suggestive of IPP revision/exchange for infectious or non-infectious reasons.
We also performed an analysis of the impact of diabetes on infectious risk in the subset of patients who underwent IPP revision surgery after their initial IPP in the dataset. We followed our patients after initial IPP placement and identified patients whose first revision surgery was for non-infectious causes. We excluded patients who first appeared in SPARCS with a revision surgery because we were unable to identify what number revision was being performed. We followed patients from their first revision surgery for non-infectious causes to determine subsequent infection rates in this cohort.
Variables Analyzed
Patient demographic information including age, race, insurance status, as well as patient comorbidities were collected. Comorbidities were assessed using the unique patient identifier codes and tracking diagnosis codes associated with hospital visits before IPP implantation. A composite comorbidity score was calculated using the Elixhauser Comorbidity Index,18 which was then modified to exclude diabetes to isolate diabetes as an independent variable. Diabetes status of the patient was determined using ICD-9-CM coding. Mean annual surgeon volume was calculated as previously described by our group.19 Procedural date cutoffs were used to define the era of IPP implantation as pre-antibiotic-coated implant era or antibiotic-coated implant era. The antibiotic-coated implant from American Medical Systems (Minneapolis, MN, USA) was introduced in 2001 and the bacterial resistant Mentor Corporation implant (Santa Barbara, CA, USA) was introduced in 2003. We therefore defined the pre-antibiotic-coated IPP era as 1996–2003 whereas 2004–2014 was classified as the antibiotic-coated IPP era.
Outcomes
The primary outcome analyzed was reoperation for infectious complications. Surgical options for infectious IPP complications included IPP explant, IPP explant with IPP replacement, or IPP explant with placement of a semi-rigid penile prosthesis. Procedural codes included Explant: ICD-9-CM 6496 or CPT 54406; Explant and IPP/semi-rigid reimplantation: ICD-9-CM 6495 or 6497 or CPT 54410 or 54411. Infectious cause as the reason for reoperation was determined using ICD-9-CM diagnosis codes associated with the reoperation visit. This included ICD-9-CM code 996.65 (infection or inflammation of genitourinary device). Patient follow-up was stopped at the time of reoperation for infection. Alternatively, if a patient underwent IPP reoperation for non-infectious complication, he was censored at that time. Patients without any evidence of reoperation were censored at the end of the study period.
Statistical Analysis
Baseline patient and surgical factors were compared between groups using chi-squared analysis for categorical variables and a Student’s t-test for continuous variables. Median values were compared using the Mann-Whitney test. Kaplan-Meier survival analyses were used to compare infection-free survival in various cohorts. Univariate and multivariable Cox proportional hazards regression analyses were then constructed to identify independent variables associated with infectious risk. All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA) with statistical significance defined as P < .05.
Results
A total of 14,969 patients were identified who underwent a primary IPP implantation from 1996–2014 with a median follow-up of 95.1 months (interquartile range [IQR]: 42.1–156.1 months). A total of 343 patients (2.3%) experienced an infection requiring surgery during the study period. This included 4.2% (217/5,200) in the pre-antibiotic-coated IPP era and 1.5% (126/8,209) in the antibiotic-coated-IPP era (P < .001). A total of 4,478 (29.9%) patients who underwent IPP placement had a preexisting diagnosis of diabetes. Patient characteristics stratified by diabetes status are shown in Table 1. Diabetic patients were slightly younger than non-diabetics and a lower proportion of diabetics were white than non-diabetics. There was a higher proportion of implants in diabetic patients in the era of antibiotic-coated IPPs (31.4% vs 27.5%, P < .001) and a slightly higher proportion of diabetics had their IPPs implanted by less-experienced surgeons. Infectious complications were experienced by 3% (133/4,478) of diabetic patients and 2% (210/10,491) of non-diabetic patients (P < .001). Of those who experienced an infection, the median time to infection was 3.0 months (IQR: 1.0–20.2) in diabetic patients and 4.5 months (IQR: 1.0–25.0) in non-diabetic patients (P = .06).
Table 1.
Patient characteristics stratified by diabetes status
| Variable | Nondiabetic N = 10,491 |
Diabetic N = 4,478 |
P value∗ |
|---|---|---|---|
| Age (mean ± SD) | 61.3 ± 10.6 | 60.0 ± 9.6 | <.001 |
| Race (%) | <.001 | ||
| White | 3,767 (42.4) | 1,458 (36.5) | |
| Non-white | 5,121 (57.6) | 2,538 (63.5) | |
| Elixhauser Comorbidity Index (%) | <.001 | ||
| <1 | 6,781 (64.6) | 3,065 (68.4) | |
| 1 | 3,145 (30.0) | 1,119 (25.0) | |
| >1 | 565 (5.4) | 294 (6.6) | |
| Insurance (%) | .001 | ||
| Private | 4,654 (57.2) | 2,077 (55.7) | |
| Medicare | 3,111 (38.2) | 1,416 (38.0) | |
| Medicaid/Other gov | 376 (4.6) | 234 (6.3) | |
| Era of initial surgery (%) | <.001 | ||
| Preantibiotic-coated IPP | 4,244 (72.5) | 1,613 (27.5) | |
| Antibiotic-coated IPP | 6,247 (68.6) | 2,865 (31.4) | |
| Annual surgeon volume (%) | .016 | ||
| 0–2 | 2,612 (24.9) | 1,135 (25.3) | |
| 3–7 | 2,557 (24.4) | 1,187 (26.5) | |
| 8–31 | 2,641 (25.2) | 1,080 (24.1) | |
| >31 | 2,681 (25.6) | 1,076 (24.0) |
IPP = inflatable penile prosthesis.
P values are derived from chi-square test (categorical variables) and t-test (continuous variables).
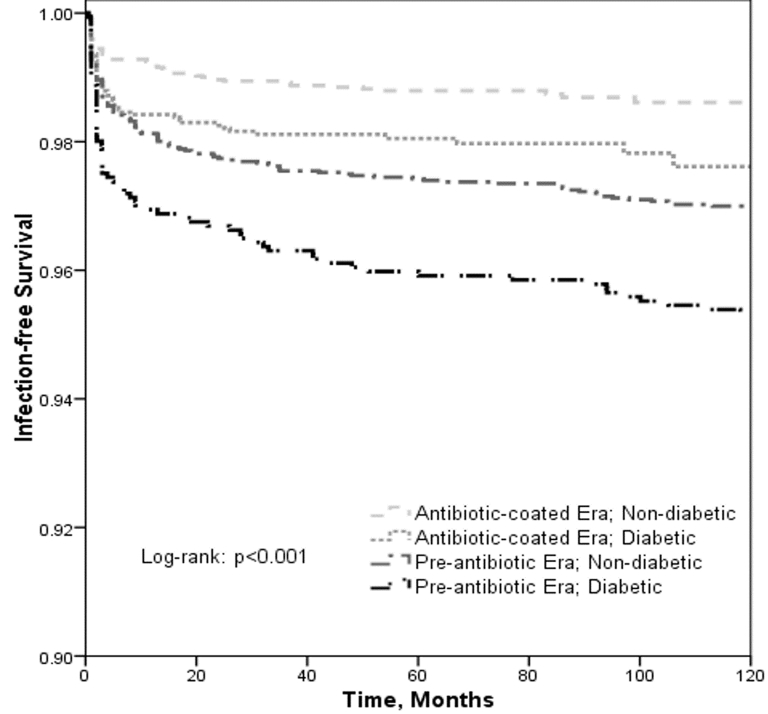
Diabetic patients had decreased implant infection-free survival both in the era before the introduction of antibiotic-coated IPPs (P = .004) as well as in the era after their widespread use (P = .003). Further, the introduction of antibiotic-coated IPPs improved the infection-free survival both in non-diabetic patients (P < .001) and in diabetic patients (P < .001). Figure 1 displays the implant infection-free survival stratified by era of implantation and diabetes status. Diabetes was associated with a significantly increased IPP infection risk on multivariable analysis controlling for age, race, comorbidities, insurance status, annual surgeon volume, and era of implantation (Hazard Ratio: 1.32, 95% CI: 1.05–1.66, P = .016) (Table 2).
Figure 1.
Kaplan-Meier Curves of IPP infection free survival stratified by era of implantation and diabetes status.
Table 2.
Multivariate Cox Proportional hazards of IPP infection
| Variable | Initial Implant Surgery N = 14,969 |
Implant Revision Surgery N = 454 |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (continuous) | 0.98 (0.97–0.99) | .003 | 1 (0.96–1.04) | .86 |
| Race (%) | ||||
| White | Ref | Ref | Ref | Ref |
| Non-white | 1.19 (0.94–1.51) | .14 | 1.1 (0.49–2.45) | .81 |
| Elixhauser Comorbidity Index∗ | ||||
| 0 | Ref | Ref | ||
| 1 | 1.04 (0.80–1.34) | .77 | 0.76 (0.29–1.99) | .58 |
| >1 | 1.28 (0.84–1.94) | .25 | 0.65 (0.09–4.96) | .68 |
| Insurance | ||||
| Private | Ref | Ref | Ref | Ref |
| Medicaid/Other gov | 1.54 (1.20–1.98) | .001 | 0.80 (0.18–3.52) | .77 |
| Medicare | 1.54 (1.04–2.28) | .032 | 0.85 (0.34–2.17) | .74 |
| Diabetes | 1.32 (1.05–1.66) | .016 | 1.27 (0.55–2.92) | .58 |
| Era: Postantibiotic-coated vs Preantiobiotic-coated | 0.46 (0.36–0.59) | <.001 | 1.16 (0.45–2.98) | .76 |
| Annual surgeon volume | ||||
| >31 | Ref | Ref | Ref | Ref |
| 0–2 | 2.41 (1.64–3.54) | <.001 | 3.18 (0.69–14.74) | .14 |
| 3–7 | 2.48 (1.69–3.64) | <.001 | 4.00 (0.86–18.58) | .08 |
| 8–31 | 2.06 (1.37–3.09) | <.001 | 2.57 (0.48–13.72) | .27 |
The bold values correspond specifically to Diabetes as the variable with infection.
HR = hazard ratio; IPP = inflatable penile prosthesis.
Excluding diabetes.
In a subset analysis, 617 patients underwent re-operation for non-infectious reasons. Of those, 454 (74%) underwent an IPP revision or replacement, including 125 diabetic patients (27.5%). Of the total revision/replacement cohort, 27 patients (5.9%) went on to experience an IPP infection, a significantly higher proportion than in the initial implantation group (P < .001). There was no notable difference between the proportion of diabetics (9/125, 7.2%) and non-diabetics (18/329, 5.5%) who experienced infectious complications after revision surgery (P = 0.49). On multivariate Cox regression analysis (Table 2), diabetes was not found to be associated with infection risk at revision (Hazard Ratio: 1.27, 95% CI: 0.55–2.92, P = .58).
Discussion
The identification of diabetes as a risk factor for IPP infection has been controversial. In this study using population-based data from a large statewide database, we identified diabetes as an independent risk factor for IPP infection after initial implantation.
There are a number of reasons why having diabetes could increase the risk of IPP infection. These include impaired wound healing caused by decreased growth factor production leading to delayed angiogenesis, and reepithelialization. This delay in wound healing may allow entry of pathogenic organisms. Additionally, diabetic patients appear to have defects in innate cellular immunologic function, thereby leading to difficulty clearing infection once present.6 Finally, diabetes leads to a hyperglycemic tissue environment that, in addition to the effects on immunity, may be ideal for bacterial growth. Compounding the inherent issues with diabetes, there are specific factors of IPP implantation in diabetic patients that may put those patients at increased risk of infection. Some have posited that impaired corporal elasticity in diabetic patients could result in increased compression of penile vasculature during implant inflation, which could theoretically predispose to penile ischemia and suboptimal delivery of innate cellular immunity and antibiotics to the penis.20
Despite the theoretical basis of diabetes as a risk factor of IPP infection, the urologic literature regarding this issue to date is mixed. In 1992, Bishop et al21 published an 18-month prospective study involving 90 patients (32 diabetic patients) undergoing penile prosthesis implantation. All infections occurred in the diabetic patients and higher glycosylated hemoglobin levels were reported to be associated with increased infection risk. Wilson et al12 performed a retrospective review of over 1,000 inflatable prosthesis implantations including >800 primary implants. In their analysis, diabetic patients had a 3% risk of infection compared with 1% in non-diabetics, though this was not found to be statistically significant. This rate increased to 18% in diabetics requiring revision surgery. Similar results have been found in multiple single-institution cohorts.13, 16 After their initial study, Wilson et al17 went on to perform a 2-year prospective study of 389 patients that included 114 diabetics. They found that infections developed in 7.5% of diabetic patients and 3.3% of non-diabetics who underwent initial IPP placement, which was once again not statistically significant (P = .09). They then analyzed their results by blood glucose control and found no increased infection risk in patients with elevated glycosylated hemoglobin levels. A follow-up of their study (included in a reply by the authors to a comment on their study), however, included 657 patients and noted a significantly increased infection rate in diabetic patients compared with non-diabetics (7.7% vs 3.3% in initial implantation, P = .036).
Even if the literature consistently and clearly reported that diabetes is a risk factor for infection, the question would remain as to whether this determination would be applicable in the modern era of penile implant surgery. The introduction of antibiotic-impregnated IPPs has led to decreased infection rate both in non-diabetics as well as in diabetic patients.22 Carson reviewed American Medical Systems (AMS; Minnetonka, MN, USA) patient information forms and found that the introduction of antibiotic coating decreased the infection rate from 1.61% to 0.68% at 6 months’ follow-up.23 A review of Coloplast (Minneapolis, MN, USA) patient information forms including >36,000 patients demonstrated a decreased infection rate with the hydrophilic implant (4.6% vs 1.4%).24 Similarly Eid et al9 performed an internal review including over 2,000 patients and found that the introduction of antibiotic coating improved infection rates from 7.0% to 2.2% using Coloplast implants and from 4.0% to 1.60% using AMS implants. It is not clear, though, if the antibiotic coating would be protective enough to negate the negative effects of diabetes on infection risk. Our study is consistent with these data because implant coating decreased the infection risk by about 50% on multivariable analysis.
Our findings are also consistent with the recent report by Mulcahy and Carson.15 They analyzed the Boston Scientific (formerly AMS) database, including >30,000 patients after initial IPP implantation and made 2 important observations: i) diabetic patients had an increased risk of infection requiring surgical intervention when compared with non-diabetics and ii) the introduction of antibiotic-coated penile implants decreased the risk of infection in diabetic patients. This study, however, was limited by its reliance on industry-generated and archived patient information forms, its restriction to prostheses from 1 manufacturer, and absence of multivariable analysis to account for patient, implant, and surgeon factors that could have impacted infection rates. In contrast, our analysis is agnostic to implant manufacturer and we employed multivariable analysis. Our findings confirm that diabetic patients in fact have an increased risk of infection requiring surgery after initial implantation with a hazard ratio of 1.32. Further, we have found that this risk remains significant even after controlling for patient characteristics, surgeon experience, and era of implantation.
Contrary to our findings regarding initial implantation, we did not find that diabetes increased infection risk after IPP revision surgery. The 5.9% overall infection rate in this population is similar to that found by Henry et al25 (5.7%) reviewing over 200 revision surgeries. They found no difference in the proportion of diabetic patients based on the infectious outcome after IPP revision. The reasons for this are unclear, but may be because of low overall infection events in our series.
Owing to the nature of this study, there are a number of limitations. First, because this study is a population-based assessment, it relies on proper diagnosis and procedural coding. Second, there were a number of factors could not be identified from this dataset that potentially affect outcomes. These include operative site scrub in the operating room, perioperative antibiotic use, and surgical approach used (subcoronal, infrapubic, or penoscrotal). The implant manufacturer is also not identified, which may be important because AMS implants are impregnated with Inhibizone, a rifampin and minocycline solution, whereas Coloplast implants are hydrophilic, which inhibits bacterial adherence and absorbs antibiotics onto its surface, allowing the surgeon to determine the antibiotic dip. To this end, the period of initiation of antibiotic coating of IPPs varied by manufacturer, as referenced earlier, which may reduce the reported impact of infection reduction. In addition, the era of antibiotic-coated IPPs referenced in this study is an estimation because the exact date of transition varied between manufacturers, as referenced earlier. Also, we were unable to address the issue of controlled vs uncontrolled diabetes. Although there are diagnosis codes that refer to diabetic sequelae, this would be an inaccurate measure of glycemic control at the time of penile implantation and we therefore did not include this in our analysis. Additionally, the severity of diabetes is not quantified because glycosylated hemoglobin levels could not be captured. Finally, because this is a state-based registry, patients who moved from New York after the IPP implantation and subsequently had surgery out of New York would not have been captured.
Despite its limitations, we believe that this study adds significant evidence to the current literature that diabetes is in fact an independent risk factor for IPP infection in patients presenting for their initial IPP. This relationship holds true regardless of surgeon experience, IPP antibiotic coating, and other patient factors.
Conclusion
In patients presenting for initial IPP, having preexisting diabetes is an independent risk factor for the development of an IPP infection. This has important implications for patient selection and counseling, and raises the question of whether this increased risk can be mitigated by optimization of glycemic control before surgery. Further study is needed to determine HbA1c level at which this risk disappears compared with those without DM.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Michael Lipsky; Ifeanyi Onyeji; Peter Stahl
-
(b)Acquisition of Data
- Michael Lipsky; Ifeanyi Onyeji
-
(c)Analysis and Interpretation of Data
- Michael Lipsky; Ifeanyi Onyeji; Ron Golan; Ricardo Munarriz; James Kashanian; Doron Stember; Peter Stahl
Category 2
-
(a)Drafting the Article
- Michael Lipsky; Peter Stahl
-
(b)Revising It for Intellectual Content
- Michael Lipsky; Ifeanyi Onyeji; Ricardo Munarriz; James Kashanian; Doron Stember; Peter Stahl
Category 3
-
(a)Final Approval of the Completed Article
- Michael Lipsky; Ifeanyi Onyeji; Ron Golan; Ricardo Munarriz; James Kashanian; Doron Stember; Peter Stahl
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: None.
References
- 1.Johannes C.B., Araujo A.B., Feldman H.A. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- 2.Selvin E., Burnett A.L., Platz E.A. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Hatzimouratidis K., Hatzichristou D. How to treat erectile dysfunction in men with diabetes: From pathophysiology to treatment. Curr Diab Rep. 2014;14:545. doi: 10.1007/s11892-014-0545-6. [DOI] [PubMed] [Google Scholar]
- 4.Montague D.K., Jarow J.P., Broderick G.A. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230–239. doi: 10.1097/01.ju.0000164463.19239.19. [DOI] [PubMed] [Google Scholar]
- 5.Walsh T.J., Hotaling J.M., Smith A. Men with diabetes may require more aggressive treatment for erectile dysfunction. Int J Impot Res. 2014;26:112–115. doi: 10.1038/ijir.2013.46. [DOI] [PubMed] [Google Scholar]
- 6.Le N.N., Rose M.B., Levinson H. Implant healing in experimental animal models of diabetes. J Diabetes Sci Technol. 2011;5:605–618. doi: 10.1177/193229681100500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato H., Nakagami G., Iwahira Y. Risk factors and risk scoring tool for infection during tissue expansion in tissue expander and implant breast reconstruction. Breast J. 2013;19:618–626. doi: 10.1111/tbj.12175. [DOI] [PubMed] [Google Scholar]
- 8.Kunutsor S.K., Whitehouse M.R., Blom A.W. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. PLoS One. 2016;11:e0150866. doi: 10.1371/journal.pone.0150866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eid J.F., Wilson S.K., Cleves M. Coated implants and “no touch” surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46% Urology. 2012;79:1310–1315. doi: 10.1016/j.urology.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 10.Mandava S.H., Serefoglu E.C., Freier M.T. Infection retardant coated inflatable penile prostheses decrease the incidence of infection: A systematic review and meta-analysis. J Urol. 2012;188:1855–1860. doi: 10.1016/j.juro.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Jarow J.P. Risk factors for penile prosthetic infection. J Urol. 1996;156:402–404. doi: 10.1097/00005392-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Wilson S.K., Delk J.R. Inflatable penile implant infection: Predisposing factors and treatment suggestions. J Urol. 1995;153:659–661. [PubMed] [Google Scholar]
- 13.Lotan Y., Roehrborn C.G., McConnell J.D. Factors influencing the outcomes of penile prosthesis surgery at a teaching institution. Urology. 2003;62:918–921. doi: 10.1016/s0090-4295(03)00665-4. [DOI] [PubMed] [Google Scholar]
- 14.Balen A., Gross M.S., Phillips E.A. Active polysubstance abuse concurrent with surgery as a possible newly identified infection risk factor in inflatable penile prosthesis placement based on a retrospective analysis of health and socioeconomic factors. J Sex Med. 2016;13:697–701. doi: 10.1016/j.jsxm.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Mulcahy J.J., Carson C.C., 3rd Long-term infection rates in diabetic patients implanted with antibiotic-impregnated versus nonimpregnated inflatable penile prostheses: 7-year outcomes. Eur Urol. 2011;60:167–172. doi: 10.1016/j.eururo.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Garber B.B., Marcus S.M. Does surgical approach affect the incidence of inflatable penile prosthesis infection? Urology. 1998;52:291–293. doi: 10.1016/s0090-4295(98)00186-1. [DOI] [PubMed] [Google Scholar]
- 17.Wilson S.K., Carson C.C., Cleves M.A. Quantifying risk of penile prosthesis infection with elevated glycosylated hemoglobin. J Urol. 1998;159:1537–1539. doi: 10.1097/00005392-199805000-00034. discussion 39-40. [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A., Steiner C., Harris D.R. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Onyeji I.C., Sui W., Pagano M.J. Impact of surgeon case volume on reoperation rates after inflatable penile prosthesis surgery. J Urol. 2017;197:223–229. doi: 10.1016/j.juro.2016.08.083. [DOI] [PubMed] [Google Scholar]
- 20.Gefen A., Chen J., Elad D. Stresses in the normal and diabetic human penis following implantation of an inflatable prosthesis. Med Biol Eng Comput. 1999;37:625–631. doi: 10.1007/BF02513358. [DOI] [PubMed] [Google Scholar]
- 21.Bishop J.R., Moul J.W., Sihelnik S.A. Use of glycosylated hemoglobin to identify diabetics at high risk for penile periprosthetic infections. J Urol. 1992;147:386–388. doi: 10.1016/s0022-5347(17)37244-0. [DOI] [PubMed] [Google Scholar]
- 22.Wilson S.K., Zumbe J., Henry G.D. Infection reduction using antibiotic-coated inflatable penile prosthesis. Urology. 2007;70:337–340. doi: 10.1016/j.urology.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Carson C.C., 3rd. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171:1611–1614. doi: 10.1097/01.ju.0000118245.66976.e1. [DOI] [PubMed] [Google Scholar]
- 24.Serefoglu E.C., Mandava S.H., Gokce A. Long-term revision rate due to infection in hydrophilic-coated inflatable penile prostheses: 11-year follow-up. J Sex Med. 2012;9:2182–2186. doi: 10.1111/j.1743-6109.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 25.Henry G.D., Donatucci C.F., Conners W. An outcomes analysis of over 200 revision surgeries for penile prosthesis implantation: A multicenter study. J Sex Med. 2012;9:309–315. doi: 10.1111/j.1743-6109.2011.02524.x. [DOI] [PubMed] [Google Scholar]