Figure 3: Molecular dynamics simulations demonstrate osmolyte-induced secondary structural stabilization of EnvZc.
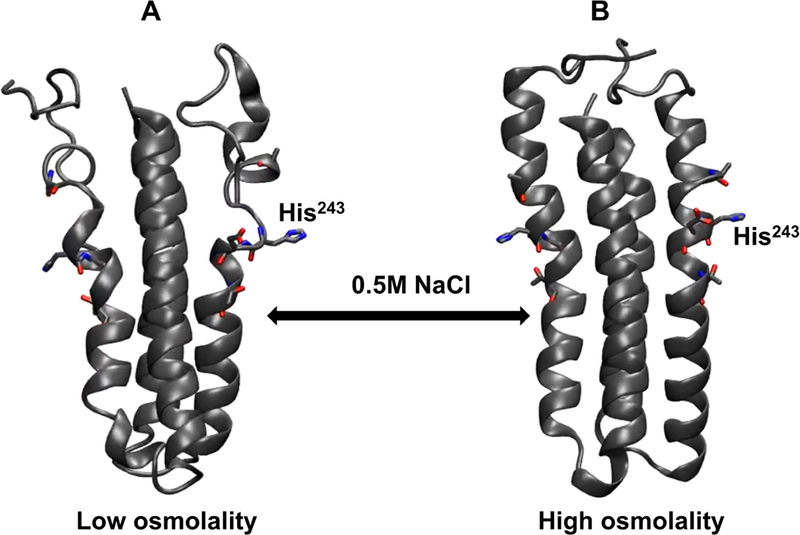
In silico models of EnvZ four helix bundle subdomain (residues 223–289) were generated under conditions mimicking low and high osmolality. It is evident from these structures that under low osmolality (left), the four-helix bundle subdomain is highly flexible particularly within the His243-containing region. In presence of high osmolality (right), there is reduced flexibility of the peptide backbone with more defined secondary structures being observed across the subdomain. These models further reinstate the model of EnvZ osmosensing proceeding through osmolyte-mediated peptide backbone stabilization of the four helix bundle.
