Figure 4: EnvZc autophosphorylation is highly sensitive to mutations in the His243 microenvironment.
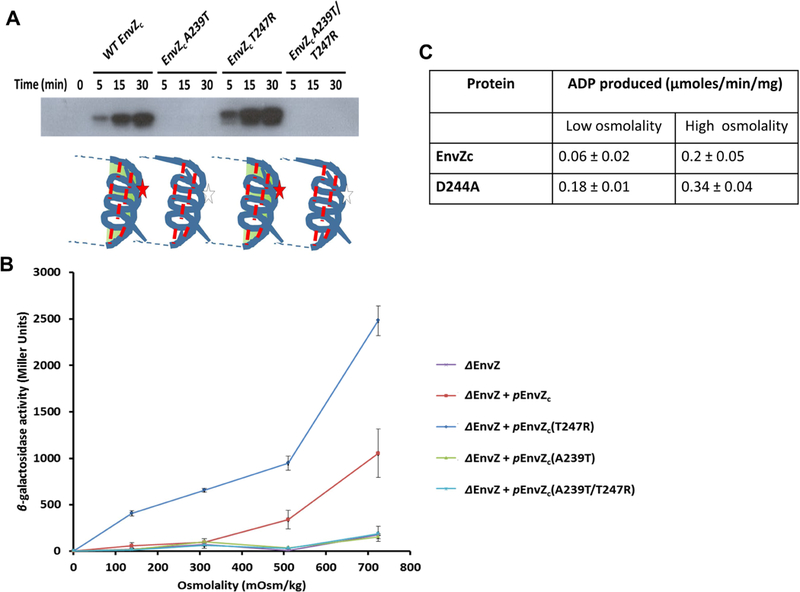
(A) Wild-type (WT), A239T, T247R and A239T/T247R EnvZc were tested for their ability to undergo autophosphorylation using a [γ−32P]-ATP kinase assay for kinase reaction times 0, 5, 15 and 30 min. WT EnvZc showed increased levels of autophosphorylation over time. The point mutants A239T and T247R revealed contrasting phenotypes with the A239T mutant showing negligible autophosphorylation while the T247R mutant showing constitutively higher levels of autophosphorylation in comparison to WT EnvZc. These results were consistent with the phenotypes observed for the same mutants in intact EnvZ (Matsuyama et al., 1986; Russo and Silhavy, 1991). The A239T/T247R EnvZc double mutant was surprisingly unable to autophosphorylate, indicating that the Ala239 residue has a significant role in modulating autophosphorylation in EnvZc (see Discussion). (B) WT EnvZc and the mutants were also examined for their ability to phosphotransfer to OmpR. Phosphorylated OmpR (OmpR~P) enhances transcription of the reporter gene fusion ompC-lacZ. In the envZ deletion (ΔenvZ) strain of E. coli PG189, a low level of ompC-lacZ expression (purple curve) is observed for cells grown in minimal A medium across a range of sucrose concentrations (0, 5, 10, and 15% (w/v), indicated as 139, 311, 510, and 724 mOsm/kg on the x-axis). When a plasmid carrying envZc (penvZc) was transformed into the ΔenvZ E. coli strain, expression of ompC-lacZ was rescued (red curve). EnvZc A239T (orange curve) and the EnvZc A239T/T247R double mutant (DM) (green curve) failed to rescue ompC-lacZ expression while EnvZc T247R (blue curve) showed higher OmpC-LacZ expression than WT EnvZc at all osmolyte concentrations. The results were reported as mean ± SEM from at least three independent measurements. (C) Autophosphorylation by EnvZ was measured using the in vitro ADP-Glo™ Kinase assay (Promega, Madison, WI), which quantifies the amount of ADP formed as a product in a kinase reaction using a luminescence-based detection method in low and high osmolality conditions as described in STAR methods.
