Figure 6.
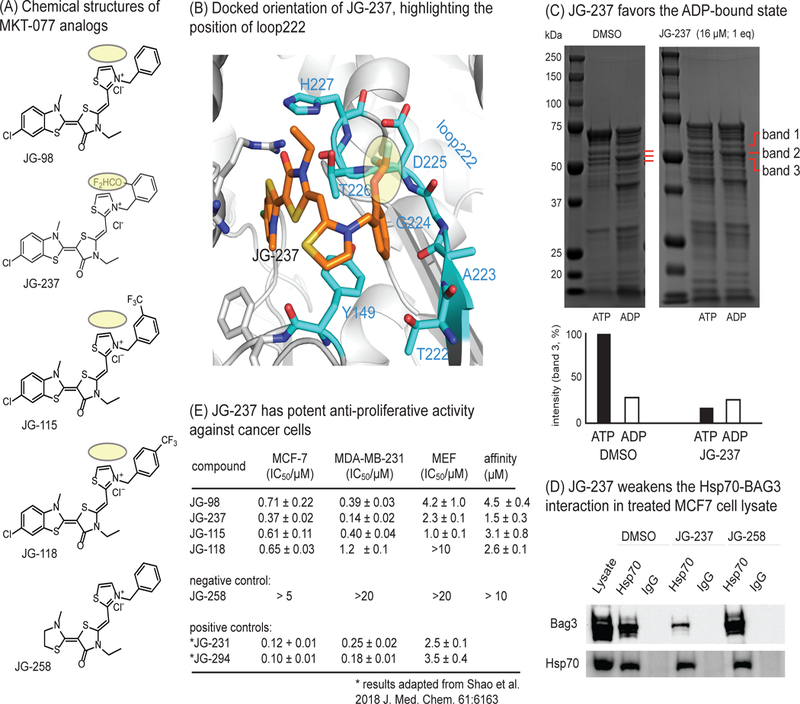
Development of an improved allosteric inhibitor of Hsc70. (A) Chemical structures of MKT-077 analogs. The bulky group addition in JG-237 is highlighted in yellow. (B) Docked structure of JG-237 bound to Hsc70’s NBD, with the position of loop222 shown. Appending a bulky group to the ortho-position of the phenyl group (yellow) is predicted to improve steric blockade of the loop222 movement. (C) Partial proteolysis confirms that JG-237 traps the ADP-like state, even when ATP is present. (D) MCF7 cell lysates were treated with JG-237 (10 µM), followed by co-immunoprecipitation with anti-Hsp70 and Western blotting for BAG3. Results are representative of duplicates. (E) JG-237 has improved anti-proliferative activity, as measured by MTT assays. Results are the average of experiment performed in triplicate and the error bars represent SEM. For reference, the activity values for positive controls (labeled with an asterisk) from a medicinal chemistry campaign are shown. The affinity of analogs for purified Hsc70 were measured by monitoring a quench in compound fluorescence upon binding. The results are average of two independent experiments performed in triplicate and error is SEM. Affinity values are typically weaker than apparent potency values for this series due to accumulation in the cancer cells. Also see Figure S5
