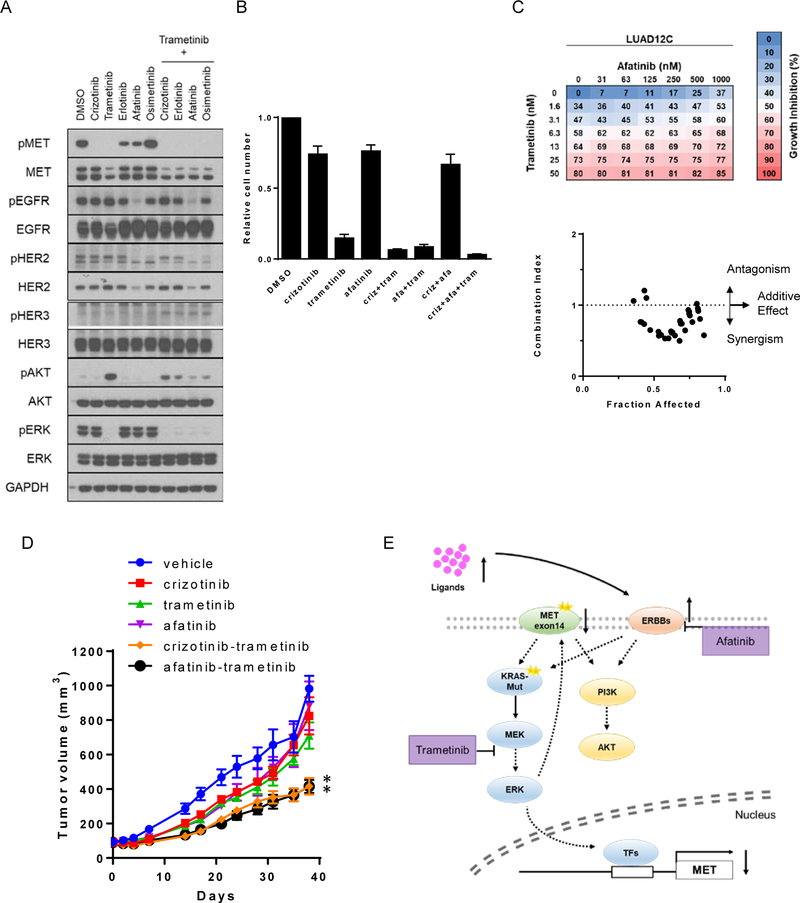
Figure 6: Trametinib synergizes with afatinib in concurrent METexon14 alteration and KRAS mutant cells.
A. LUAD12C cells were treated with crizotinib (1 μM), trametinib (25 nM), erlotinib (1 μM), afatinib (1 μM), osimertinib (1 μM), or a combination of them for 48 hours. Lysates were then subjected to immunoblotting. B. A total 3 × 104 of LUAD12C cells were plated in 6-well plates, and treated with DMSO, crizotinib (200nM), trametinib (5nM), afatinib (100 nM), or a combination of them for 11 days. Relative cell numbers are shown. Each condition was assayed in duplicate determinations in 3 independent experiments and error bar represent mean ± SE. C. LUAD12C cells were treated with a combination of trametinib and arfatinib for 96 hours. Cell viability was determined by AlamarBlue. Data represent the mean value of growth inhibition ratio at each concentration of the drugs in three independent experiments. Dot plot indicates the combination index and fraction affected (inhibition ratio) of various drug concentration. D. LUAD12C cells were implanted into a subcutaneous flank of NSG mice. When tumors reached approximately 100 mm3, mice were treated with vehicle or 25 mg/kg crizotinib, 1 mg/kg trametinib, 12.5 mg/kg afatinib, or a combination of them 4 days a week. Tumor volume was determined on the indicated days after the onset of treatment. Data represent mean ± SE (n = 5). *p<0.05, compared to trametinib-treated group. E. A strategy for the treatment of METex14 and KRAS-mutant lung cancer.