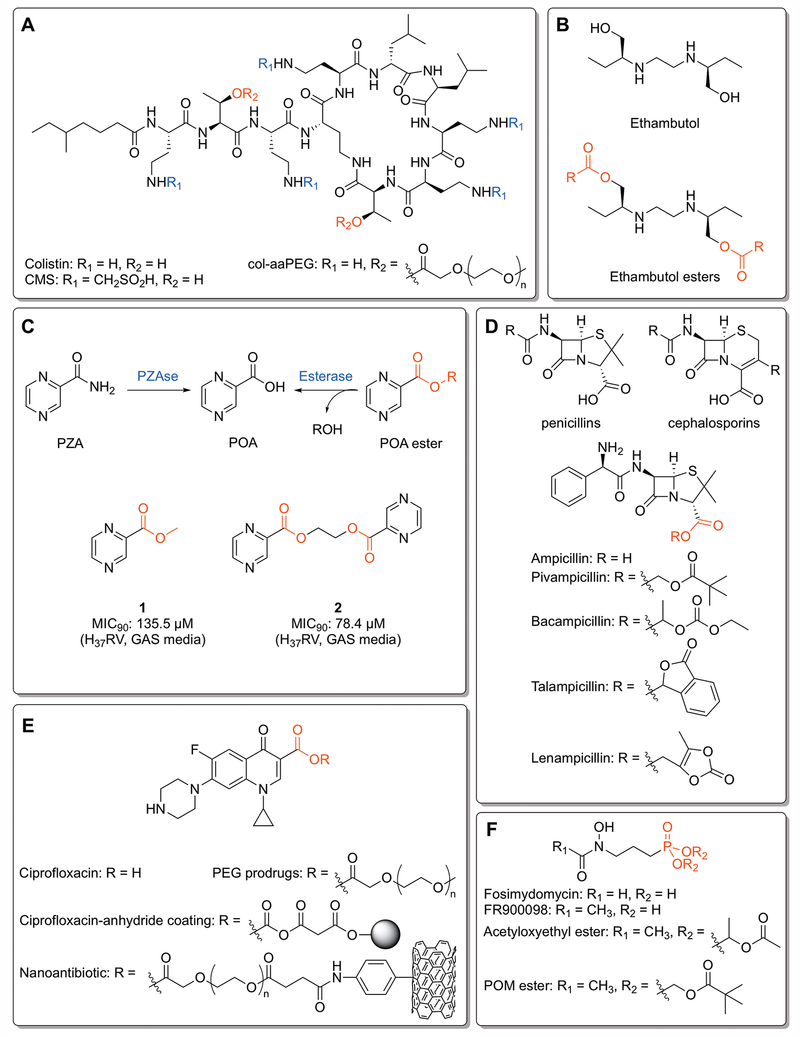
Figure 3:
Classic, clinical, and pre-clinical ester prodrugs. A) Colistin, colistin methanesulfonate (CMS),[Bergen et al. 2006] and the mono-aaPEG polymer (col-aaPEG).[Zhu et al. 2017] The PEG esters are hydrolyzed by plasma esterases at 37°C to yield colistin. B) Ethambutol and ethambutol esters. Ester prodrugs are inactive against mycobacteria until hydrolyzed by an esterase. R groups include alkyl chains, cycloalkyl groups and branched alkyl functionalities.[Larsen et al. 2017] C) The pyrazinamide (PZA) and pyrazinoic acid (POA) ester activation pathways. POA esters demonstrate activity against resistant strains of mycobacteria that lack pyranizamidase (PZAse), instead being activated by internal esterases. The “duplicated” prodrug 2 shows increased efficacy over 1 due to increased intracellular concentrations of POA.[Segretti et al. 2016] D) Core structures of the penicillins and cephalosporins, along with ampicillin and its ester prodrugs. The different ester moieties are designed to improve lipophilicity and oral bioavailability. [Bodin et al. 1975; Clayton et al. 1976; Sakamoto et al. 1984; Sjövall et al. 1978] E) Ciprofloxacin and various ester prodrugs and conjugates. PEGlyated prodrugs are designed to disrupt crystallinity and enhance solubility.[Assali et al. 2016] The ciprofloxacin-anhydride coating is engineered to be cleaved by extracellular esterases,[Komnatnyy et al. 2014] and the nanoantibiotic is designed to be cleaved by internal esterases following disruption of the bacterial cell wall by the carbon nanotube.[Assali et al. 2017] F) Fosimydomycin, FR900098, and two FR900098 phosphonate esters. The acetyloxyethyl ester demonstrates enhanced activity against malaria due to increased oral absorption,[Phillips et al. 2015] while the pivaloyloxymethyl (POM) ester activates FR900098 against Mtb.[Uh et al. 2011]