Abstract
Objectives
Peripheral inflammation has been associated with multiple psychiatric disorders, particularly with depression. However, findings remain inconsistent and unreproducible, most likely due to the disorder’s heterogeneity in phenotypic presentation. Therefore, in the present study, in an effort to account for inter-individual differences in symptom severity, we utilised a dimensional approach to assess the relationships between a broad panel of inflammatory cytokines and key psychiatric symptoms (i.e., depression, anhedonia, anxiety, fatigue, and suicidality) in adolescents across psychiatric disorders. We hypothesised that only anhedonia—reflecting deficits of reward function—will be associated with inflammation.
Methods
Participants were 54 psychotropic medication-free adolescents with diverse psychiatric conditions and 22 healthy control (HC) adolescents, ages 12–20. We measured 41 cytokines after in-vitro lipopolysaccharide stimulation. Mann-Whitney U and Spearman correlation tests examined group comparison and associations, respectively, while accounting for multiple comparisons and confounds, including depression severity.
Results
There were no group differences in cytokine levels. However, as hypothesised, within the psychiatric group, only anhedonia was associated with 19 cytokines, including hematopoietic growth factors, chemokines, and pro-inflammatory cytokines.
Conclusions
Our findings suggest that general inflammation may induce reward dysfunction, which plays a salient role across psychiatric conditions, rather than be specific to one categorical psychiatric disorder.
Keywords: inflammation, cytokines, anhedonia, adolescent, reward
Introduction
Adolescence is a developmental period notable for its increased prevalence of psychiatric conditions (Merikangas et al. 2010). Although the underlying mechanisms are not fully understood, inflammatory processes have been implicated, particularly in depression (Felger and Lotrich 2013; Gabbay et al. 2009b; Irwin and Miller 2007), but also across psychiatric conditions both in paediatric and adult populations (Furtado and Katzman 2015; Gabbay et al. 2009a; Gabbay et al. 2009c; Michopoulos et al. 2017; Mitchell and Goldstein 2014; Munkholm et al. 2013). Hence, inflammation does not seem to have diagnostic specificity, which may be due to high diagnostic comorbidity, shared etiological pathways, and/or overlapping symptoms across disorders. The non-specific association between inflammation and psychiatric conditions might be even more prominent in adolescents in light of the rapid brain changes in certain regions (e.g., frontal-striatal) (Brenhouse and Schwarz 2016).
Addressing the above phenomena, several studies have examined the relationships between inflammation, brain functions, and associated neuropsychiatric symptoms, as opposed to categorical psychiatric diagnoses (e.g., Capuron and Castanon 2017; Miller et al. 2013). Converging data suggest that inflammation may target the brain’s reward circuitry, which is known to be impaired across psychiatric conditions (Whitton et al. 2015). It has been hypothesised that inhibition of reward function by inflammation occurs in order to conserve energy needed to facilitate the healing process (Aubert 1999; De La Garza 2005; Felger and Miller 2012). Supporting this theory, immunotherapy with interferon alpha (IFN-α) in humans induces anhedonia—the decreased capacity to experience pleasure, known to reflect deficits of reward function—along with other “flu-like’” symptoms (Capuron and Miller 2004). Further, a randomised trial with the immune activator lipopolysaccharide (LPS) and a placebo (saline) in healthy individuals found that exposure to LPS induced motivational changes, as measured by a behavioural task (Lasselin et al. 2017). Neuroimaging work, using PET and fMRI modalities, adds to this literature by documenting the specific effects of inflammatory processes within the dopaminergic reward circuitry (Capuron et al. 2007; Capuron et al. 2012; Eisenberger et al. 2010; Juengling et al. 2000). A similar phenomenon has also been reported in non-human primates; for example, a study in rhesus monkeys demonstrated that chronic administration of IFN-α resulted in decreased dopaminergic neurotransmission in association with anhedonia-like behaviour (Felger et al. 2013). Similarly, cross-sectional studies have shown that patients with the melancholic subtype of major depressive disorder, characterised by high anhedonia, have elevated levels of certain pro-inflammatory cytokines (interleukin [IL]-6, IL-1β, and IL-1 receptor antagonist [ra]), compared to both healthy controls and patients with non-melancholic major depressive disorder (Dunjic-Kostic et al. 2013; Kaestner et al. 2005; Rush et al. 2016). Consistent with this, a recent fMRI study documented a negative correlation between C-reactive protein—a marker of overall inflammation—and connectivity within reward-related brain regions in adults with depression; this study further showed that this relationship was, in turn, related to higher levels of anhedonia (Felger et al. 2016). Taken together, these data suggest that inflammation may affect the reward circuitry rather than be specific to one psychiatric condition. However, no studies have examined relationships between peripheral inflammation and clinical manifestations reflecting reward function, such as anhedonia severity, in adolescents.
Therefore, in the current study, we sought to assess relationships between a broad profile of inflammatory cytokines and anhedonia in adolescents. Our sample included psychotropic medication-free adolescents with diverse psychiatric symptoms and healthy control (HC) adolescents. We elected not to focus on one specific categorical diagnosis since anhedonia and other behavioural dimensions are salient across psychiatric conditions and therefore may share the same aetiology. To better quantify the immune system, peripheral blood mononuclear cells (PBMCs) were stimulated with LPS, a bacterial endotoxin and an immune activator. Building upon findings to date, hypotheses were that: a) adolescents with psychiatric symptoms would exhibit higher levels of inflammatory cytokines compared to HCs; and b) in light of specific relationships between immune reactivity and reward processes, levels of cytokines would be associated with anhedonia severity, but not depression, suicidality, anxiety, or fatigue severity, all of which are symptoms often associated with anhedonia but do not reflect specific deficits of reward. We limited this latter hypothesis to the psychiatric group, as the HC group was not expected to exhibit a range of severity on the studied clinical measures.
Methods
Participants and Clinical Procedure
Adolescents, ages 12–20, were recruited in the greater New York City area. Participants under age 18 provided written assent, and a parent or guardian gave written informed consent; participants 18 years and older provided written informed consent. The study was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai.
Adolescents were evaluated for lifetime history of Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) psychiatric disorders to assess study eligibility. On a separate visit, occurring within two weeks of the diagnostic evaluation, participants underwent a fasting blood draw and completed clinical questionnaires. A urine toxicology test and pregnancy test (in females) were also administered, and vitals (i.e., blood pressure, pulse, weight, and height) were taken. Adolescents with a current or past DSM-IV diagnosis of schizophrenia, pervasive developmental disorder, or substance use disorder were excluded. Additional exclusion criteria for HC adolescents were any current or past DSM-IV diagnosis and/or psychiatric treatment. Participants were also excluded if they had taken any psychotropic medication in the thirty days prior to the blood draw, had taken any immune-affecting medications or herbal supplements (e.g., steroids, non-steroidal anti-inflammatory drugs, and omega-3 fatty acids) in the two weeks prior to the blood draw, or if they had a positive urine toxicology or pregnancy test. Finally, we excluded all participants with a history of any chronic inflammatory conditions or who had had an inflammatory illness (e.g., cold, flu) in the two weeks prior to the blood draw.
Clinical Measures
Diagnoses.
DSM-IV symptoms and diagnoses (at a clinical and sub-clinical level) were obtained by a trained licensed psychiatrist or clinical psychologist using the Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version for School Aged Children (K-SADS-PL) (Kaufman et al. 1997). The interview was administered to adolescent participants, as well as to a parent or guardian when the participant was under age 18. To enhance diagnostic reliability, evaluations were discussed between the interviewing clinician and the Principal Investigator (a board-certified child and adolescent psychiatrist).
Depression severity.
Participants self-reported on their depression severity using the Beck Depression Inventory-II (BDI-II) (Beck et al. 1996). The BDI-II is a 21-item self-report scale that assesses symptoms and features of depression over the previous two weeks. The BDI-II has been validated for its high internal consistency in clinical (Krefetz et al. 2002) and non-clinical (Osman et al. 2008) adolescent populations. Reliability was excellent in the present sample (α = .94).
Anhedonia.
Participants completed the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al. 1995), a self-report measure of anhedonia severity. The SHAPS is a measure of consummatory reward across four positive valence subcategories: hobbies, social interactions, sensory experiences, and food. Higher total scores reveal greater difficulty experiencing pleasure in these areas. The SHAPS has been validated in clinical and non-clinical populations of adolescents and adults (Franken et al. 2007; Leventhal et al. 2015) and is currently considered the “gold standard” for measuring anhedonia in depression (Leventhal et al. 2015; Rizvi et al. 2016). Reliability was good in the present sample (α = .89).
Anxiety.
The Multidimensional Anxiety Scale for Children (MASC) (March et al. 1997) was used as a measure of anxiety severity. The MASC is a 39-item, self-report scale that assesses anxiety across four main symptom areas: 1) Physical Symptoms; 2) Social Anxiety; 3) Harm Avoidance; and 4) Separation Anxiety. Higher scores indicate greater anxiety severity. Deemed reliable and validated in both clinical and non-clinical populations (March et al. 1997), the MASC is able to discriminate between anxiety and depression in participants (Rynn et al. 2006). The full MASC score was utilised for the present analyses. Reliability was excellent in the present sample (α = .92).
Suicidality.
Participants were administered the Beck Scale for Suicidal Ideation (BSS) (Beck et al. 1988), a 21-item self-report measure used to evaluate suicidality. The first 19 items assess for suicidal ideation in the past two weeks, and items 20 and 21 assess for lifetime suicidality. The BSS has been validated for use with adolescent populations (Steer et al. 1993). Reliability was excellent in the present sample (α = .98).
Fatigue.
Fatigue was assessed using the 18-item self-report PedsQL™ Multidimensional Fatigue Scale (MFS) (Varni and Limbers 2008). The MFS is designed to measure fatigue over the past month in paediatric and young adult populations across three domains: general fatigue, sleep/rest fatigue, and cognitive fatigue. The total score was used in the present analyses with lower scores indicating greater fatigue. The MFS has demonstrated good to excellent validity and reliability, spanning age and sex groups in paediatric and young adult populations with a variety of conditions (Panepinto et al. 2014; Tomlinson et al. 2013; Varni and Limbers 2008). Reliability was excellent in the present sample (α = .92).
Multiplex analysis
All blood samples were collected between 9:00 and 10:00 AM after an overnight fast (≥ 12 hours). Samples were processed within 20 minutes of collection and stored at −80º C. Detailed sera cytokine profile was measured using a Luminex-200 system and the XMap Platform (Luminex Corporation). Acquired fluorescence data were analysed by the xPONENT software. Levels of inflammatory cytokines were determined in duplicate 25 μL volumes of plasma or serum using the multiplex cytokine panels (Multiplex High Sensitivity Human Cytokine Panel, Millipore Corp.). Assays were performed by laboratory staff at Mount Sinai’s Human Immune Monitoring Center (HIMC) who were blind to participants’ clinical status. The assays included 41 cytokines (listed in Supplementary Tables 1 and 2). To assess participants’ functional immune responses, whole blood was stimulated for 6 hours with toll-like receptor 4 (TLR4) agonist LPS, a well-established and potent immune activator (Lu et al. 2008). Following the 6-hour exposure, supernatants were harvested and analysed by Luminex for the panel of cytokines. The potency of LPS was authenticated on a quarterly basis using the in-house reference PBMCs collected from a healthy donor in the Multiplex cytokine assay.
Median fluorescence intensity (MFI) values were not transformed into absolute concentration values for the current analyses. The use of MFI values provides direct comparison of the data without introducing any bias for the samples with very low or high values in relation to the provided standard curves (Breen et al. 2015; Breen et al. 2016).
Statistical procedures
All statistical analyses were performed in SPSS, version 22. According to Shapiro-Wilk tests, all inflammatory cytokines were found to have a non-normal distribution in our sample, necessitating the use of non-parametric analyses. Prior to our main analyses, we examined group differences in demographic variables (i.e., age, sex, and ethnicity), as well as in the relationships between demographic and clinical variables, to detect any potential confounds. We also examined body mass index (BMI) as a potential confound, in light of findings that adiposity increases inflammatory responses to stress (McInnis et al. 2014) and may mediate the relationship between depression and inflammation (Miller et al. 2003).
We first ran Mann-Whitney U tests comparing adolescents with psychiatric symptoms and HC adolescents on MFI values for each of the 41 inflammatory cytokines following LPS stimulation (post-LPS). Given the large number of comparisons, a False Discovery Rate (FDR) adjustment (Benjamini and Hochberg 1995), corrected to p < .05, was applied.
Next, for the psychiatric group, we conducted Spearman correlations to examine the associations between clinical measures (BDI, SHAPS, MASC, BSS, MFS) and post-LPS MFI values. For these analyses, BMI, age, and sex were included as covariates, given the wide age range of participants in our study and the compelling literature linking BMI to inflammation (Miller et al. 2003) and sex differences in immune response (Oertelt-Prigione 2012; Roved et al. 2017). For each set of correlations, an FDR adjustment, corrected to p < .05, was applied. To control for the potential confounding role of overall depression severity, for correlations with the SHAPS, MASC, BSS, and MFS, the total BDI score was included as a covariate (i.e., partial correlation). Importantly, in order to limit shared variance between the clinical measures and the items on the BDI that correspond to these constructs, we removed these items prior to controlling for the BDI total score. Specifically, for the partial correlations between SHAPS and post-LPS MFI values, we removed BDI Item 2, assessing anhedonia (“difficulty having fun”), from the total BDI score. Similarly, for the partial correlations between BSS and post-LPS MFI values, we removed BDI Item 13 (“suicidal ideation”) from the total BDI score. Finally, for the partial correlations between the MFS and post-LPS MFI values, we removed BDI Item 6 (“excessive fatigue”) from the total BDI score. There is no item assessing anxiety on the BDI, so for the partial correlations between the MASC and post-LPS MFI values, we controlled for the total BDI score with no items removed.
Results
Participant characteristics
The sample consisted of 76 adolescents, ages 12–20 (56.6% female), including 54 adolescents with DSM-IV psychiatric symptoms and 22 HC adolescents. The adolescents with psychiatric symptoms met criteria for one or more DSM-IV diagnoses on a clinical or sub-clinical level. The majority of participants (92% of the full sample, and 97% of the psychiatric group) were in an advanced puberty (i.e., Tanner stages 4 and 5; as assessed by self-report based on sex-specific drawings). Table 1 provides demographic and clinical characteristics for the sample. Adolescents with psychiatric symptoms had significantly higher scores on all clinical measures. The groups did not differ on any demographic variables or in BMI.
Table 1.
Demographic and diagnostic data
| Psychiatric Group (n = 54) |
Healthy Control Group (n = 22) |
|
|---|---|---|
| Age in years [Mean ± SD] | 15.37 ± 2.23 | 15.45 ± 2.77 |
| Sex female [%] | 55.6% | 59.1% |
| Race [%] | ||
| White | 44.4% | 50.0% |
| Black | 35.2% | 36.4% |
| Asian | 3.7% | 0.0% |
| More than one race/other | 16.7% | 13.6% |
| Ethnicity [%] | ||
| Hispanic | 46.3% | 27.3% |
| Non-Hispanic | 53.7% | 72.7% |
| Body mass index [Mean ± SD] | 26.16 ± 7.68 | 23.12 ± 5.47 |
| Clinical measures [Mean ± SD] | ||
| BDI | 14.27 ± 11.41 | 1.18 ± 1.74*** |
| SHAPS | 24.22 ± 6.06 | 17.10 ± 3.63*** |
| MASC | 41.90 ± 19.34 | 24.05 ± 10.98*** |
| BSS | 2.51 ± 4.66 | 0.00 ± 0.00*** |
| MFS | 31.91 ± 13.13 | 13.63 ± 9.63*** |
| DSM-IV lifetime diagnoses [%] a,b | ||
| Depressive disorder c | 59.3% | |
| Bipolar spectrum disorder d | 7.4% | |
| Anxiety disorder e | 51.9% | |
| Attention deficit hyperactivity disorder | 42.6% | |
| Behavioural disorder f | 20.4% | |
| Other disorder g | 9.3% | |
| Number of lifetime diagnoses [%] | ||
| One | 31.5% | |
| Two | 33.3% | |
| Three or more | 35.2% |
BDI = Beck Depression Inventory; SHAPS = Snaith-Hamilton Pleasure Scale; MASC = Multidimensional Anxiety Scale for Children; BSS = Beck Scale for Suicidal Ideation; MFS = PedsQL™ Multidimensional Fatigue Scale.
includes subthreshold presentations;
some participants have more than one disorder;
major depressive disorder (n = 28), dysthymic disorder (n = 5), depressive disorder not otherwise specified (NOS; n = 3);
bipolar II disorder (n = 1), bipolar disorder NOS (n = 2), mood disorder NOS (n = 1);
social anxiety disorder (n = 11), generalised anxiety disorder (n = 13), panic disorder (n = 1), separation anxiety disorder (n = 2), specific phobia (n = 3), obsessive-compulsive disorder (n = 2), posttraumatic stress disorder (n = 6), anxiety disorder NOS (n = 1);
oppositional defiant disorder (n = 5), behavioural disorder NOS (n = 1);
eating disorder NOS (n = 1), adjustment disorder (n = 1), enuresis (n = 1), Tourette’s disorder (n = 1).
Significant group differences at p < .001
In adolescents with psychiatric disorders, BDI, BSS, SHAPS, and MFS scores were moderately intercorrelated, but MASC scores were only associated with the BDI. No demographic variables were associated with these clinical measures. Despite this, as noted above, we took a conservative approach and controlled for age, sex, and BMI in analyses examining correlations between clinical measures and post-LPS MFI values.
One participant in the psychiatric group was removed from the cytokine analyses given extreme levels (i.e., greater than 2 standard deviations above the mean) of a number of the 41 cytokines measured.
Group differences in inflammatory cytokines
HC adolescents and adolescents with psychiatric symptoms did not differ on post-LPS MFI values for any of the examined inflammatory cytokines at the FDR corrected threshold (see Supplementary Table 1).
Correlations between inflammatory cytokines and clinical measures
The SHAPS was the only clinical measure associated with inflammatory cytokines in the psychiatric group at the FDR corrected thresholds. As presented in Table 2, controlling for BMI, age, sex, and depression severity (as described above), SHAPS scores were significantly positively correlated with post-LPS values of the following 19 cytokines at the FDR corrected threshold: FGF-2, Flt3-L, Fractalkine, G-CSF, GM-CSF, IL-1α, IL-2, IL-3, IL-4, IL-7, IL-9, IL-10, IL-12p40, IL-12p70, IL-15, IL-17α, MCP-3, TNF-β, VEGF.
Table 2.
Significant relationshipsa between inflammatory cytokines following LPS stimulation and clinical measures within the psychiatric group
| SHAPSb | ||
|---|---|---|
| Cytokine | Correlation (ρ) | p-value |
| FGF-2 | .421 | .004 |
| Flt3-L | .513 | .000 |
| Fractalkine | .572 | .000 |
| G-CSF | .570 | .000 |
| GM-CSF | .488 | .001 |
| IL-1α | .436 | .002 |
| IL-2 | .349 | .018 |
| IL-3 | .387 | .008 |
| IL-4 | .379 | .009 |
| IL-7 | .479 | .001 |
| IL-9 | .419 | .004 |
| IL-10 | .334 | .023 |
| IL-12p40 | .372 | .011 |
| IL-12p70 | .542 | .000 |
| IL-15 | .548 | .000 |
| IL-17α | .370 | .011 |
| MCP-3 | .350 | .017 |
| TNF-β | .371 | .011 |
| VEGF | .506 | .000 |
SHAPS = Snaith-Hamilton Pleasure Scale; FGF = fibroblast growth factor; Flt3-L = fms-like tyrosine kinase-3 ligand; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; IL = interleukin; MCP = monocyte chemotactic protein; TNF = tumour necrosis factor; VEGF = vascular endothelial growth factor.
at the FDR correction threshold;
controlling for BDI with anhedonia item removed, body mass index, age, and sex.
Discussion
This is the first study to our knowledge to examine peripheral inflammatory cytokines and anhedonia severity as well as other related behavioural dimensions in adolescents with diverse psychiatric conditions. Contrary to our first hypothesis, our findings did not suggest that psychotropic medication-free adolescents with psychiatric conditions exhibit overall increased inflammation when compared to healthy adolescents. However, in line with our second hypothesis, across clinical measures, only anhedonia severity showed associations with multiple cytokines, lending support to the idea that inflammation is associated specifically with reward system deficits. We discuss our findings below.
In contrast to a number of studies in individuals with depression and other psychiatric conditions, (Dowlati et al. 2010; Furtado and Katzman 2015; Miller et al. 2009; Munkholm et al. 2013; Young et al. 2014) including from our laboratory (Gabbay et al. 2009a; Gabbay et al. 2009b; Gabbay et al. 2009c), the current study failed to detect any compelling group differences in levels of inflammatory cytokines between adolescents with psychiatric conditions and healthy control adolescents. Similarly, a number of other studies in both adult and youth populations did not find significant group differences (Byrne et al. 2013; Cilan et al. 2012; Einvik et al. 2012; Kim et al. 2014; Marques-Deak et al. 2007), including a recent large-scale study examining peripheral inflammation in psychotropic medication-free adults with major depressive disorder and healthy controls (Cassano et al. 2017). The latter study also failed to detect relationships with depression illness severity (Cassano et al. 2017). Such inconsistencies across studies can be attributed to the high inter-individual variability of clinical presentation, even in a homogenous clinical diagnostic category such as major depressive disorder (Gabbay et al. 2015). Indeed, as mentioned above, in our prior published paper (Gabbay et al. 2009b), we did report increased levels of pro-inflammatory cytokines and a pro-inflammatory state in adolescents with major depressive disorder compared to healthy controls. However, this prior sample of major depressive disorder had high illness severity, which may have reflected higher anhedonia. This is in contrast to the current sample that consisted of participants with diverse psychiatric conditions with high variability of anhedonia severity (as presented in Table 1). The lack of detected group differences in the current study supports our overall approach that a dimensional investigative method may be preferred to better detect neurobiological underpinnings of behavioural constructs (Insel 2014).
As hypothesised, our data demonstrated that only anhedonia was associated with levels of inflammatory cytokines, and all correlations were positive. This finding is consistent with past research demonstrating associations between inflammation and deficits in reward-related neural correlates in adult populations (Capuron et al. 2012; Eisenberger et al. 2010; Felger et al. 2016). Our finding is also supported by a recent randomised trial with LPS and placebo (saline) in healthy adult individuals, which showed that LPS induces motivational changes as measured by a behavioural task (Lasselin et al. 2017).
Although no other studies have examined relationships between peripheral inflammatory markers and anhedonia in adolescent populations, our research group and others have found associations between the inflammatory kynurenine pathway (KP) and anhedonia (Gabbay et al. 2012; Gabbay et al. 2010a; Gabbay et al. 2010b; Savitz et al. 2015). The KP metabolises tryptophan into several neurotoxins hypothesised to induce central nervous system (CNS) alterations seen in depression and is induced by pro-inflammatory cytokines. Our findings include increased KP activation in adolescents with the melancholic subtype of depression (Gabbay et al. 2010a), characterised by high levels of anhedonia, as well as relationships between KP metabolites and dimensional measures of anhedonia severity (Gabbay et al. 2012). More recently, in an fMRI study, we documented associations between KP metabolite blood levels and connectivity within the reward and salience networks in adolescents with depression (DeWitt et al. 2017). Therefore, our present finding of relationships among a wide variety of cytokine levels and anhedonia severity adds additional evidence that inflammation may have a specific role in the reward circuitry in youth.
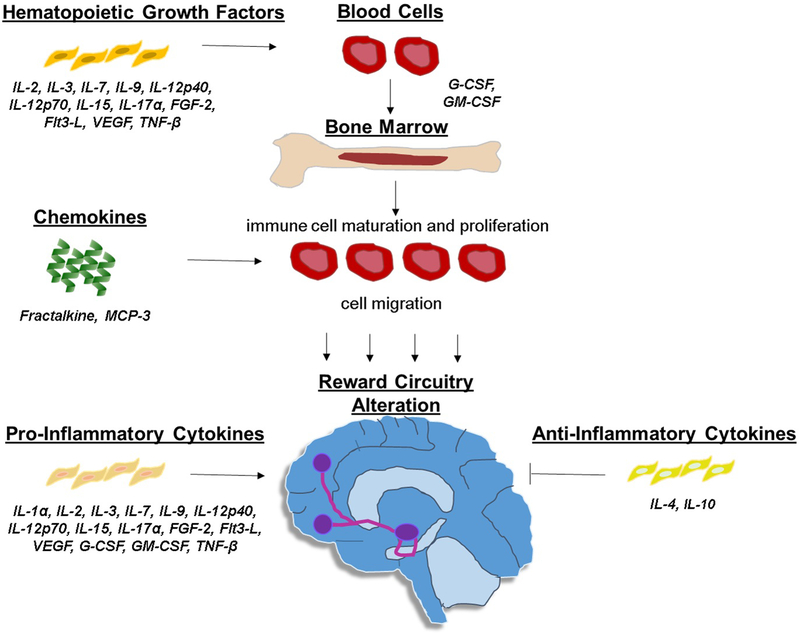
Despite a conservative statistical approach with a correction for multiple comparisons while controlling for several potential covariates (i.e., depression severity, age, sex, BMI), 19 cytokines were found to be associated with anhedonia. This result suggests that generalised and complex immunological reactions may play a role in the induction of reward dysfunction. Our detected associations include several classes of cytokines that are involved in multiple stages of an immune reaction including: a) hematopoietic growth factors (IL-2, IL-3, IL-7, IL-9, IL-12p40, IL-12p70, IL-15, IL-17α, FGF-2, Flt3-L, VEGF, G-CSF, GM-CSF, TNF-β), which induce proliferation and maturation of blood cells including the initiation of an immune reaction by stimulating the production of immune cells from the bone marrow (e.g., G-CSF, GM-CSF) b) chemokines (Fractalkine, MCP-3), which induce immune cell migration, c) pro-inflammatory cytokines (IL-1α, IL-2, IL-3, IL-7, IL-9, IL-12p40, IL-12p70, IL-15, IL-17α, FGF-2, Flt3-L, VEGF, G-CSF, GM-CSF, TNF-β), which are part of complex inflammatory pathways, and d) anti-inflammatory cytokines (IL-4, IL-10), which regulate the inflammatory immune response, interacting with cytokine inhibitors and receptors. Importantly, some cytokines have overlapping immune functions (see Figure 1 illustrating the function of these cytokines within the immune system). Due to technological advances, our approach of assessing multiple cytokines simultaneously has only been recently utilised, and very few papers have examined them in relation to behavioural domains in clinical populations. However, converging evidence has associated these cytokines with the CNS as well as with depression symptomatology (Clark-Raymond and Halaris 2013; Dahl et al. 2014; Gaughran et al. 2006; Shelton et al. 2011; Simon et al. 2008; Zhao et al. 2015).
Figure 1. Function of our detected cytokines within the immune system.
Flt3-L = fms-like tyrosine kinase 3-ligand; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; IL = interleukin; MCP = monocyte chemotactic protein; TNF = tumour necrosis factor; VEGF = vascular endothelial growth factor.
Within the hematopoietic growth factor family, both FGF-2 and VEGF have a role in promoting neuronal survival within the fronto-striatal region (Reuss and von Bohlen und Halbach 2003; Sharma et al. 2016). While we would have expected a negative correlation between these neurotrophic factors and anhedonia, the positive relationship may represent a compensatory mechanism to the overall heightened immune system activation. Within the chemokine family, fractalkine is found throughout the brain with preclinical studies implicating it with anhedonia. Specifically, fractalkine receptor knockout mice did not exhibit any anhedonic like symptoms post stress, suggesting that fractalkine may impact the reward circuitry (Winkler et al. 2017).
Although others have found relationships between cytokine levels and overall depression and anxiety severity (Vogelzangs et al. 2016), such a relationship was not present among our sample of youth with diverse psychiatric conditions. However, both anxiety and depression severity are associated with disturbances of reward processes, and these prior studies did not assess such relationships with anhedonia severity (Morris et al. 2015).
Our results must be interpreted in light of several limitations. First, the combination of a relatively small sample size with a stringent threshold for determining statistical significance (given multiple comparisons) may have meant that smaller effects of inflammation on psychiatric symptomatology were not detected. However, at both p = .05 and at the FDR-corrected threshold, anhedonia was the only clinical characteristic that showed relationships with multiple cytokines. Regardless, further studies are needed using larger samples of youth with psychiatric symptoms to confirm the present findings. As a second limitation, in an effort to examine relationships with adolescent reward function, we utilised self-report measures of anhedonia. Although the SHAPS and other clinical measures of anhedonia have been shown to be associated with reward system neurocircuitry (Felger et al. 2016), the measures may not completely map onto decreased reward-related brain function. Future studies should utilise neuroimaging approaches to more accurately capture the neural underpinnings of reward anticipation and attainment (Bradley et al. 2017). Finally, our analyses did not account for other biological triggers (e.g., physical exercise, menstrual cycle stage) which may impact cytokine levels (Allen et al. 2015; Whitcomb et al. 2014). Importantly, our approach of in vitro LPS stimulation minimizes the effect of these factors as it assesses the immune system subsequent to a biological stressor.
In summary, this study supports the notion that peripheral inflammation may be associated with disturbances in reward function in adolescents rather than underlie a specific psychiatric category. Since psychiatric manifestation in youth can evolve into different psychiatric conditions at adulthood, there is a critical need to develop biomarkers that would predict course of illness and progression. Our finding of associations between cytokine levels and anhedonia is an important development in this direction. This study underscores the importance of incorporating dimensional analyses to address the heterogeneous nature of psychiatric conditions and overlapping symptomatology among disorders. Future studies should utilise longitudinal analyses to assess whether such relationships predict illness progression while assessing reward function objectively with neuroimaging or reward tasks.
Supplementary Material
Acknowledgements
This study was supported by grants from the National Institute of Health (NIH) MH101479 and MH095807 (PI: Gabbay).
Footnotes
Statement of Interest
The authors declare no conflict of interest.
References
- Allen J, Sun Y, Woods JA. 2015. Exercise and the Regulation of Inflammatory Responses. Prog Mol Biol Transl Sci 135:337–54. [DOI] [PubMed] [Google Scholar]
- Aubert A 1999. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev 23(7):1029–36. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, Ranieri WF. 1988. Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol 44(4):499–505. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological 57(1):289–300. [Google Scholar]
- Bradley KAL, Case JAC, Freed RD, Stern ER, Gabbay V. 2017. Neural correlates of RDoC reward constructs in adolescents with diverse psychiatric symptoms: A Reward Flanker Task pilot study. J Affect Disord 216:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EJ, Polaskova V, Khan A. 2015. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine 71(2):188–98. [DOI] [PubMed] [Google Scholar]
- Breen EJ, Tan W, Khan A. 2016. The Statistical Value of Raw Fluorescence Signal in Luminex xMAP Based Multiplex Immunoassays. Sci Rep 6:26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Schwarz JM. 2016. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev 70:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek JM, Woods MJ, Trinder J, Allen NB. 2013. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav Immun 34:164–75. [DOI] [PubMed] [Google Scholar]
- Capuron L, Castanon N. 2017. Role of Inflammation in the Development of Neuropsychiatric Symptom Domains: Evidence and Mechanisms. Curr Top Behav Neurosci 31:31–44. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. 2004. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry 56(11):819–24. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. 2007. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 32(11):2384–92. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. 2012. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69(10):1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano P, Bui E, Rogers AH, Walton ZE, Ross R, Zeng M, Nadal-Vicens M, Mischoulon D, Baker AW, Keshaviah A et al. 2017. Inflammatory cytokines in major depressive disorder: A case-control study. Aust N Z J Psychiatry 51(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilan H, Oguzhan N, Unal A, Turan T, Koc AN, Sipahioglu MH, Utas C, Oymak O. 2012. Relationship between depression and proinflammatory cytokine levels in hemodialysis patients. Ren Fail 34(3):275–8. [DOI] [PubMed] [Google Scholar]
- Clark-Raymond A, Halaris A. 2013. VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res 47(8):1080–7. [DOI] [PubMed] [Google Scholar]
- Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, Brundin L, Andreassen OA. 2014. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45:77–86. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd. 2005. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev 29(4–5):761–70. [DOI] [PubMed] [Google Scholar]
- DeWitt SJ, Bradley KA, Lin N, Yu C, Gabbay V. 2017. A pilot resting-state functional connectivity study of the kynurenine pathway in adolescents with depression and healthy controls. J Affect Disord 227:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67(5):446–57. [DOI] [PubMed] [Google Scholar]
- Dunjic-Kostic B, Ivkovic M, Radonjic NV, Petronijevic ND, Pantovic M, Damjanovic A, Poznanovic ST, Jovanovic A, Nikolic T, Jasovic-Gasic M. 2013. Melancholic and atypical major depression--connection between cytokines, psychopathology and treatment. Prog Neuropsychopharmacol Biol Psychiatry 43:1–6. [DOI] [PubMed] [Google Scholar]
- Einvik G, Vistnes M, Hrubos-Strom H, Randby A, Namtvedt SK, Nordhus IH, Somers VK, Dammen T, Omland T. 2012. Circulating cytokine concentrations are not associated with major depressive disorder in a community-based cohort. Gen Hosp Psychiatry 34(3):262–7. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. 2010. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68(8):748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 21(10):1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. 2013. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH. 2012. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 33(3):315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL et al. 2013. Chronic Interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38(11):2179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P. 2007. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). J Affect Disord 99(1–3):83–9. [DOI] [PubMed] [Google Scholar]
- Furtado M, Katzman MA. 2015. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res 229(1–2):37–48. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Coffey BJ, Guttman LE, Gottlieb L, Katz Y, Babb JS, Hamamoto MM, Gonzalez CJ. 2009a. A cytokine study in children and adolescents with Tourette’s disorder. Prog Neuropsychopharmacol Biol Psychiatry 33(6):967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Babb J, Liebes L. 2012. The possible role of the kynurenine pathway in anhedonia in adolescents. J Neural Transm 119(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, Klein RG. 2015. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. J Child Adolesc Psychopharmacol 25(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ. 2009b. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord 115(1–2):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ. 2009c. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol 19(4):423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. 2010a. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry 51(8):935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. 2010b. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry 34(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. 2006. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull 70(3):221–7. [DOI] [PubMed] [Google Scholar]
- Insel TR. 2014. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry 171(4):395–7. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. 2007. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 21(4):374–83. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. 2000. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 152(4):383–9. [DOI] [PubMed] [Google Scholar]
- Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, Arolt V, Cassens U, Rothermundt M. 2005. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J Affect Disord 87(2–3):305–11. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36(7):980–8. [DOI] [PubMed] [Google Scholar]
- Kim JW, Szigethy EM, Melhem NM, Saghafi EM, Brent DA. 2014. Inflammatory markers and the pathogenesis of pediatric depression and suicide: a systematic review of the literature. J Clin Psychiatry 75(11):1242–53. [DOI] [PubMed] [Google Scholar]
- Krefetz DG, Steer RA, Gulab NA, Beck AT. 2002. Convergent validity of the Beck Depression Inventory-II with the Reynolds Adolescent Depression Scale in psychiatric inpatients. J Pers Assess 78(3):451–60. [DOI] [PubMed] [Google Scholar]
- Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, Paues-Goranson S, Axelsson J, Dantzer R, Lekander M. 2017. Lipopolysaccharide alters motivated behavior in a monetary reward task: a randomized trial. Neuropsychopharmacology 42(4):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Unger JB, Audrain-McGovern J, Sussman S, Volk HE, Strong DR. 2015. Measuring anhedonia in adolescents: A psychometric analysis. Journal of Personality Assessment 97(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42(2):145–51. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. 1997. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36(4):554–65. [DOI] [PubMed] [Google Scholar]
- Marques-Deak AH, Neto FL, Dominguez WV, Solis AC, Kurcgant D, Sato F, Ross JM, Prado EB. 2007. Cytokine profiles in women with different subtypes of major depressive disorder. J Psychiatr Res 41(1–2):152–9. [DOI] [PubMed] [Google Scholar]
- McInnis CM, Thoma MV, Gianferante D, Hanlin L, Chen X, Breines JG, Hong S, Rohleder N. 2014. Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun 42:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. 2010. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49(10):980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. 2017. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 42(1):254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. 2013. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30(4):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65(9):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. 2003. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun 17(4):276–85. [DOI] [PubMed] [Google Scholar]
- Mitchell RH, Goldstein BI. 2014. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J Am Acad Child Adolesc Psychiatry 53(3):274–96. [DOI] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, Yaroslavsky I, Kovacs M, Rottenberg J. 2015. Reward learning in pediatric depression and anxiety: preliminary findings in a high-risk sample. Depress Anxiety 32(5):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkholm K, Brauner JV, Kessing LV, Vinberg M. 2013. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res 47(9):1119–33. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S 2012. The influence of sex and gender on the immune response. Autoimmun Rev 11(6–7):A479–85. [DOI] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Williams JE, Bailey J. 2008. Psychometric properties of the Beck Depression Inventory-II in nonclinical adolescent samples. J Clin Psychol 64(1):83–102. [DOI] [PubMed] [Google Scholar]
- Panepinto JA, Torres S, Bendo CB, McCavit TL, Dinu B, Sherman-Bien S, Bemrich-Stolz C, Varni JW. 2014. PedsQL Multidimensional Fatigue Scale in sickle cell disease: feasibility, reliability, and validity. Pediatr Blood Cancer 61(1):171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. 2003. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res 313(2):139–57. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. 2016. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev 65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roved J, Westerdahl H, Hasselquist D. 2017. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav 88:95–105. [DOI] [PubMed] [Google Scholar]
- Rush G, O’Donovan A, Nagle L, Conway C, McCrohan A, O’Farrelly C, Lucey JV, Malone KM. 2016. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J Affect Disord 205:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynn MA, Barber JP, Khalid-Khan S, Siqueland L, Dembiski M, McCarthy KS, Gallop R. 2006. The psychometric properties of the MASC in a pediatric psychiatric sample. J Anxiety Disord 20(2):139–57. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R. 2015. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40(2):463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AN, da Costa e Silva BF, Soares JC, Carvalho AF, Quevedo J. 2016. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J Affect Disord 197:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. 2011. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16(7):751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. 2008. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol 18(3):230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 167(1):99–103. [DOI] [PubMed] [Google Scholar]
- Steer RA, Kumar G, Beck AT. 1993. Self-reported suicidal ideation in adolescent psychiatric inpatients. J Consult Clin Psychol 61(6):1096–9. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, Hinds PS, Ethier MC, Ness KK, Zupanec S, Sung L. 2013. Psychometric properties of instruments used to measure fatigue in children and adolescents with cancer: a systematic review. J Pain Symptom Manage 45(1):83–91. [DOI] [PubMed] [Google Scholar]
- Varni JW, Limbers CA. 2008. The PedsQL Multidimensional Fatigue Scale in young adults: feasibility, reliability and validity in a University student population. Qual Life Res 17(1):105–14. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW. 2016. Cytokine production capacity in depression and anxiety. Transl Psychiatry 6(5):e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb BW, Mumford SL, Perkins NJ, Wactawski-Wende J, Bertone-Johnson ER, Lynch KE, Schisterman EF. 2014. Urinary cytokine and chemokine profiles across the menstrual cycle in healthy reproductive-aged women. Fertil Steril 101(5):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. 2015. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 28(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler Z, Kuti D, Ferenczi S, Gulyas K, Polyak A, Kovacs KJ. 2017. Impaired microglia fractalkine signaling affects stress reaction and coping style in mice. Behav Brain Res 334:119–128. [DOI] [PubMed] [Google Scholar]
- Young JJ, Bruno D, Pomara N. 2014. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord 169:15–20. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang Y, Pan F. 2015. The effects of EGb761 on lipopolysaccharide-induced depressive-like behaviour in C57BL/6J mice. Cent Eur J Immunol 40(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.