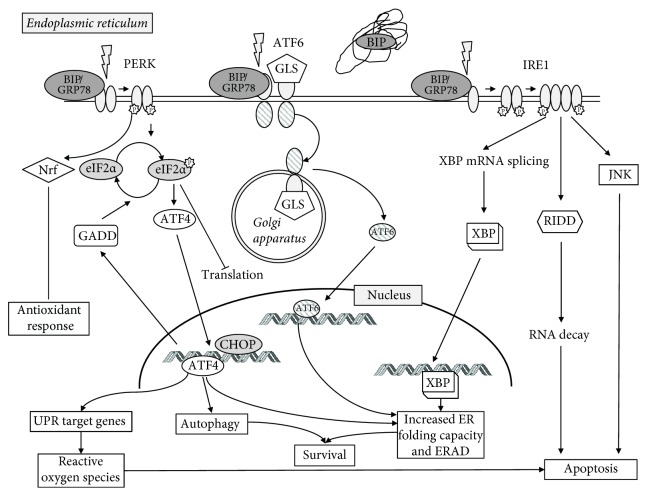
Figure 1.
The UPR signaling cascade. UPR pathways are activated through competitive binding of the chaperone immunoglobulin heavy-chain-binding protein (BiP) also known as glucose-regulated protein 78 (GRP78) to the receptors. Accumulation of misfolded or unfolded proteins in the endoplasmic reticulum (ER) leads to the dissociation of BiP from 3 transducers: PERK (double-stranded RNA-activated protein kinase-like ER kinase), ATF6 (activating transcription factor 6), and IRE1 (inositol-requiring enzyme). Upon activation, PERK phosphorylates and deactivates the eukaryotic initiation factor (eIF2α), which results in an increased level of ATF4. This triggers the activation of C/EBP homologous protein (CHOP). Subsequently, DNA damage-inducible protein transcript (GADD) expression is also elevated, what negatively regulates eIF2α phosphorylation and restores translation. While initially contributing to cellular survival in conditions of ER stress, PERK is considered proapoptotic due to strong induction of CHOP in chronic or terminal ER stress. PERK also regulates several transcription factors including NRF-2 that upregulate the antioxidant response and ATF4 which can lead to both protective and apoptotic signaling. Upon activation, ATF6 is released from BiP that is trafficked to the Golgi apparatus (it consists of two Golgi localization signals, GLS) and cleaved by the proteases into two subunits. Then it translocates to the nucleus where it is the promoter region of UPR target genes termed the endoplasmic reticulum stress element (ERSE), activating genes responsible for the components of the UPR response and leads to the induction of molecular chaperones (e.g., GRP78, Grp94, and calreticulin, as well as CHOP and XBP1). The various ER chaperones are part of a protective adaptive response that regulates protein folding and other components of the UPR. ATF6 is primarily considered prosurvival due to its role in promoting the transcription of chaperones and XBP1. IRE-1 activation is responsible for in the unconventional splicing of XBP-1 mRNA. Spliced XBP-1 encodes a transcription factor that activates the expression of UPR genes, such as chaperones and ER-associated degradation proteins (ERAD). These include the activation of the cell death machinery, degradation of ER-localized mRNAs that encode secreted and membrane proteins through the RIDD (regulated Ire1-dependent decay) pathway, and induction of autophagosomes. This signaling cascade increases the folding capacity of the ER and causes degradation of misfolded proteins. IRE1 is mainly considered as a prosurvival pathway, but it also can contribute to apoptosis through the activation of JNK-dependent pathway.