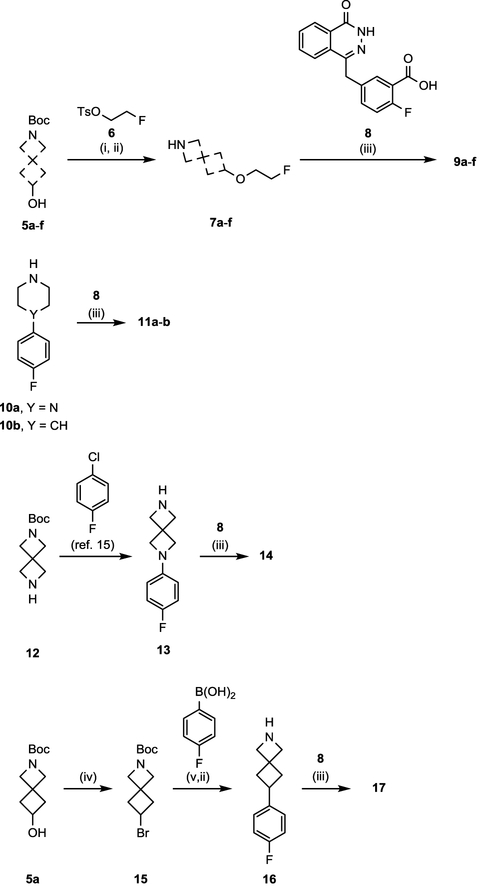
Scheme 1. Synthesis of PARPi 9a-f, 11a-b, 14, and 17a.
aReagents and conditions: (i) Respective hydroxyl amine (5a-f), NaH, 6, DMF, rt, 2 h; (ii) TFA, DCM, rt, 3 h; (iii) 8, respective free-amine, HOBt hydrate, EDC HCl, TEA, THF, 60 °C, 12 h. (iv) 5a, PPh3, CBr4, THF, rt; (v) 15, (4-fluorophenyl)boronic acid, NiI2, trans-2-aminocyclohexanol hydrochloride, NaHMDS, 2-methyl-2-butanol 80 °C, 12 h.