Figure 1.
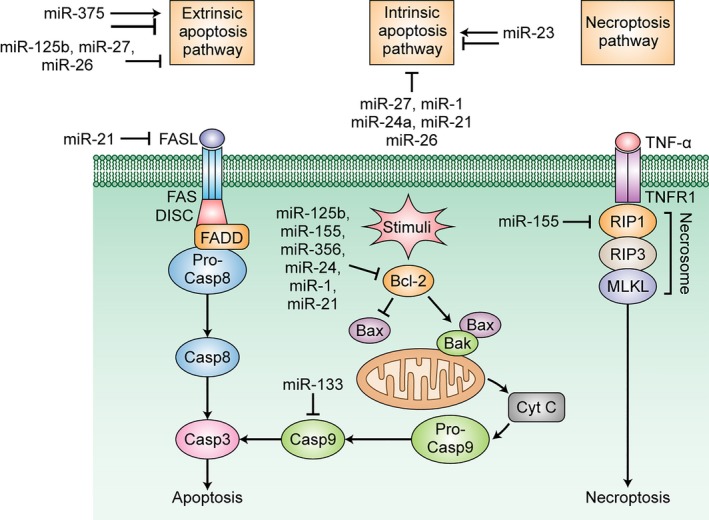
Cell death pathways currently implicated in motor neuron cell death in ALS and related miRNAs. (A) Extrinsic apoptosis. Fas ligand (FASL) activation of the cognate FAS death receptor induces intracellular formation of the death‐inducing signalling complex (DISC), which in turn interacts with Fas‐associated protein with death domain (FADD) through their death effector domains, inducing the recruitment and dissociation of pro‐caspase‐8 from the DISC. Activated caspase‐8 initiates the caspase cascade and leads to apoptosis through caspase‐3 activation. miR‐375 can promote the extrinsic pathway by various mechanisms. miR‐375, miR‐125b, and miR‐27a/b can inhibit the extrinsic pathway by silencing p53. miR‐21 can inhibit the extrinsic pathway by targeting FasL. (B) Intrinsic apoptosis. Following cytotoxic stimuli, pro‐apoptotic BH3‐only proteins silence the anti‐apoptotic protein BCL2, effectively allowing BAX and BAK to dimerize. This dimerization makes the outer mitochondrial membrane permeable and allows cytochrome_c (cyt_c) release into the cytoplasm. The cyt_c binding to apoptotic protease activating factor‐1 (Apaf‐1) prompts formation of the apoptosome, which activates caspase‐9 and the caspase cascade, in which apoptosis is ultimately driven by caspase‐3 activation. MiR‐125b, miR‐155, miR‐365, miR‐24, miR‐1, and miR‐21 promote apoptosis by silencing BCL2. MiR‐133a inhibits apoptosis by silencing caspase‐9. MiR‐23, miR‐27, miR‐1, miR‐21, and miR‐26 inhibit intrinsic apoptosis, and miR‐23 has also pro‐apoptotic properties. (C) Necroptosis. Necroptosis is favoured by low levels of caspase‐8, combined with sufficient concentrations of receptor‐interacting protein 3 (RIP3) and mixed lineage kinase domain like pseudokinase (MLKL). Following tumour necrosis factor receptor 1 (TNFR1) stimulation by tumour necrosis factor (TNF), RIP1 interacts with RIP3, and its phosphorylation is activated MLKL. They form a complex called the necrosome, which causes cell membrane rupture. miR‐155 inhibits necroptosis by silencing RIP1
