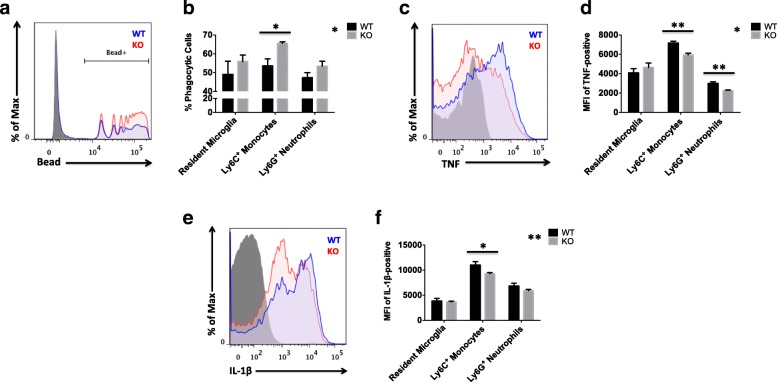
Fig. 2.
Lack of CD200R1-mediated immune inhibitory signaling differentially affects myeloid cell functions in the ischemic brain. Representative histograms illustrate the functional differences in phagocytic activity (a) and TNF-α (b) and IL-1β (c) production between infiltrating monocytes of CD200R1-KO (red) and WT littermate controls (blue) at 72 h after ischemic stroke. Cell-specific FMO controls were used to determine positive gating (shaded gray). Results from the ex vivo phagocytosis bead assay were quantified for resident microglia (CD45intCD11b+Ly6C−) and brain-infiltrating monocytes (CD45hiCD11b+Ly6C+Ly6G−) and neutrophils (CD45hiCD11b+Ly6C+Ly6G+) in each group (b; N = 6/group). The mean fluorescence intensities (MFI) of TNF-positive (d) and IL-1β-positive (f) myeloid cells were measured to assess differences in the relative protein expression level of these subsets between groups (N = 6 mice/genotype/surgery group). Asterisks adjacent to group labels designate an effect of genotype by two-way ANOVA. KO knockout, WT wild-type, SEM standard error of mean. *p < 0.05; **p < 0.01