Abstract
To explore the clinical significance of preoperative serum CEA, CA125, and CA19-9 levels in predicting the resectability of cholangiocarcinoma. Patients with cholangiocarcinoma diagnosed by radiologic examination and admitted to the Second Affiliated Hospital of Harbin Medical University from September 1, 2011, to November 30, 2017, were retrospectively included. The relationship between the preoperative serum CEA, CA125, and CA19-9 levels and the resectability of cholangiocarcinoma was analyzed by receiver operating characteristic (ROC) curve, as well as the best cut-off point. A total of 112 met the inclusion criteria. In 50 patients with radical surgeries, the levels of preoperative serums CEA, CA125, and CA19-9 were 5.0 ± 13.9 ng/mL, 15.3 ± 11.8 U/mL, and 257.5 ± 325.6 U/mL, respectively, which were lower than those in patients with unresectable tumor. Based on the ROC curve, the ideal CA19-9 cut-off value was determined to be 1064.1 U/mL in prediction of resectability, with a sensitivity of 53.2%, a specificity of 94.0%, and the area under the ROC curve of 0.73 (P < 0.05). The cut-off value of CA125 was 17.8 U/mL with a sensitivity of 72.6%, a specificity of 78.0%, and the area under the ROC curve of 0.81 (P < 0.05). The cut-off value of CEA was 2.6 ng/mL with a sensitivity of 79.0%, a specificity of 48.0%, and the area under the ROC curve of 0.66 (P < 0.05). In addition to this, we found that using the combination of three tumor markers could improve the value in predicting resectability of cholangiocarcinoma. In summary, this study suggested that the preoperative serum CEA, CA125, and CA19-9 levels can help predict the resectability of cholangiocarcinoma.
1. Introduction
Cholangiocarcinoma is the most common primary tumor of the biliary tract, with a poor prognosis at the advanced stages [1–3]. The morbidity and mortality of cholangiocarcinoma have increased over the past 40 years, especially in Asia [4]. Currently, radical resection is accepted widely as one of the preferred treatment options for cholangiocarcinoma [5, 6]. However, cholangiocarcinoma is closely associated with adjacent structures, such as the portal vein and hepatic artery [7], and it is characterized by an infiltrative growth [8, 9]. In addition, due to its insidious onset and high malignancy, it is often found in advanced stages at diagnosis [10]. Therefore, the surgical resection rate of cholangiocarcinoma is low [11, 12]. It is of increasing clinical importance to evaluate the resectability of the tumor before operation.
Currently, the assessment of resectability of cholangiocarcinoma is based on a combination of clinical, radiological, and biochemical approaches [13, 14]. Cholangiocarcinoma grows along the wall of the bile duct or the connective tissue around the bile duct, forming no nodule or mass in many cases, thus displaying no mass shadow in radiological examinations [15, 16], whereas surgical exploration is invasive and may result in a huge financial burden on patients.
There is no biomarker currently available for the resectability of cholangiocarcinoma that is sufficiently sensitive and specific. As key markers for the diagnosis of gastrointestinal malignancies and predicting their prognosis, carcinoembryonic antigen (CEA), carbohydrate antigen (CA)125, and CA19-9 have also been widely used to predict the resectability of tumors [17–19]. At present, they are also important markers in the diagnosis of cholangiocarcinoma. It was reported that preoperative serum CA19-9 level was positively associated with tumor stage. However, the increase in CA19-9 may be caused by cholangitis or obstructive jaundice. Thus, it may not be accurate to use CA19-9 alone in clinical practice. Mucins have been associated with human malignant tumors. CA125 is currently considered to be MUC16, and its amino acid sequence has some properties of mucin molecules, which may have better clinical application in adenocarcinoma [20]. CEA levels are not related to serum bilirubin levels and may be effective in predicting surgical resection rates. In general, it remains unclear whether their expression levels are valuable in determining the resectability of cholangiocarcinoma. In this study, we retrospectively analyzed the preoperative serum CEA, CA125, and CA19-9 levels in 112 patients with cholangiocarcinoma who were treated in our center from 2011 to 2017 and explored their clinical value in determining the resectability of cholangiocarcinoma.
2. Materials and Methods
We retrospectively analyzed clinically diagnosed or pathologically confirmed cholangiocarcinoma patients who had been admitted to the Second Affiliated Hospital of Harbin Medical University from September 1, 2011, to November 30, 2017. The clinical diagnosis was mainly based on clinical symptoms, imaging findings [computed tomography (CT), ultrasonography, magnetic resonance imaging, positron emission tomography–CT, and endoscopic ultrasonography], and tumor markers including CEA, CA125, and CA19-9 [21]. The patients were staged according to the American Joint Committee on Cancer (AJCC) Staging System. Tumor resectability was confirmed by intraoperative exploration; all patients were from more than two chief physicians to determine whether to perform radical surgery. In addition, a tumor was confirmed to be unresectable if radiological examination revealed the presence of hepatic metastasis or other distant metastasis. In order to make sure the decision is uniformed, most of the patients were selected from nearly three years and treated by three chief physicians. Patients with incomplete clinical data were excluded.
2.1. Determination of Serum CA19-9, CA125, and CEA Levels
Before treatment, 5 mL peripheral blood was extracted from the peripheral vein, and plasma and albumin were isolated by centrifugation at 2000 × g for 15 min. The CA125 and CA19-9 levels were determined by radioimmunoassay [22, 23], with a normal upper limit of 35 U/mL and 37 U/mL, respectively. The CEA level was determined by ELISA [24], with a normal upper limit of 5 ng/mL.
2.2. Statistical Analysis
Statistical analysis was performed using SPSS 22.0. Numerical data were presented as the mean ± standard deviation. A two-tailed P value < 0.05 was considered statistically significant. The optimal cut-offs for CEA, CA125, and CA19-9 in determining the resectability were analyzed using the receiver operating characteristic (ROC) curves.
3. Results
In total, 112 cholangiocarcinoma patients (66 men and 46 women; male/female ratio 1.43; average age 62.5 years) were retrieved. The disease was pathologically confirmed in 72 cases and clinically diagnosed in 40 cases. Although pathological diagnosis was more reliable, clinical diagnosis was acceptable based on the patients' symptoms and accessory examinations.
3.1. General Clinical Features of Cholangiocarcinoma Patients
Cholangiocarcinoma was resectable in 50 cases (44.6%) and unresectable in 62 cases (55.4%). The lesions were located in hepatic segments in 16 cases, at the hepatic hilum in 35 cases, and in the distal bile duct in 61 cases. There were 50 cases of radical resection and 22 cases of palliative resection, which were also histologically classified as highly (n = 24), moderately (n = 28), or poorly (n = 20) differentiated. The rest of the patients were treated by endoscopic or ultrasound intervention, so there was no pathological diagnosis. The AJCC staging results were as follows: two stage I, resection rate 100%; 44 stage II, resection rate 93.2%; 24 stage III, resection rate 29.2%; and 42 stage IV, all of which were unresectable. The sizes of resectable tumor tissue were determined consistently by a trained pathologist, and those of the unresectable group were determined consistently by a trained radiologist. The average tumor diameter was 2.3 ± 0.9 cm in the resectable group, which was significantly smaller than that of the unresectable group (4.5 ± 1.6 cm, P < 0.05, Table 1).
Table 1.
Relationship between clinical features and resectability of cholangiocarcinoma patients.
| Features | Resectable (n = 50) | Unresectable (n = 62) | Total (n = 112) | P value |
|---|---|---|---|---|
| Age (yr) | 60.9 ± 7.7 | 63.7 ± 1 0.0 | 62.5 ± 9.1 | |
| Sex (n) | 0.172 | |||
| Men | 33 | 33 | 66 | |
| Women | 17 | 29 | 46 | |
| Tumor location (n) | 0.029 | |||
| Intrahepatic | 4 | 12 | 16 | |
| Hilar | 12 | 23 | 35 | |
| Distal | 34 | 27 | 61 | |
| Differentiation (n) | 0.003 | |||
| High | 20 | 4 | 24 | |
| Moderate | 22 | 6 | 28 | |
| Poor | 8 | 12 | 20 | |
| Tumor diameter (cm) | 2.3 ± 0.9 | 4.5 ± 1.6 | 3.5 ± 1.3 | |
| AJCC stage (n) | 0.000 | |||
| I | 2 | 0 | 2 | |
| II | 41 | 3 | 44 | |
| III | 7 | 17 | 24 | |
| IV | 0 | 42 | 42 | |
| CEA (ng/mL) | 5.0 ± 13.9 | 19.1 ± 69.2 | 12.8 ± 52.6 | |
| CA125 (U/mL) | 15.3 ± 11.8 | 48.8 ± 58.7 | 33.9 ± 47.3 | |
| CA19-9 (U/mL) | 257.5 ± 325.6 | 730.1 ± 527.5 | 519.1 ± 505.4 | |
| Total bilirubin (μmol/L) | 187.1 ± 121.7 | 219.3 ± 174.9 | 204.9 ± 153.5 |
AJCC Cancer Staging Manual 7th edition. Patients were divided into the resectable and unresectable groups; some patients in the unresectable group were diagnosed according to imaging findings and therefore had no data on pathological stage or AJCC stage.
3.2. Serum CEA, CA125, and CA19-9 Levels in Determining Cholangiocarcinoma Resectability
Table 2 shows the multivariate logistic regression models for predicting the resectability of cholangiocarcinoma. Multivariate logistic regression analysis for predicting the resectability of cholangiocarcinoma showed that the serum levels of CEA, CA125, and CA19-9 had a better predictive value in radical resection.
Table 2.
Multivariate logistic regression models for predicting the resectability of cholangiocarcinoma.
| Observation | Predicted value | ||
|---|---|---|---|
| Radical resection | Nonradical resection | Correct percentage | |
| Radical resection | 43 | 7 | 86.0% |
| Nonradical resection | 25 | 37 | 59.7% |
| Overall percentage | 60.7% | 39.3% | 71.4% |
The accuracy of the model in predicting surgical resectability is higher, reaching 86.0% (P < 0.05).
Serum CEA, CA125, and CA19-9 levels in the resectable group (n = 50) were 5.0 ± 13.9 ng/mL, 15.3 ± 11.8 U/mL, and 257.5 ± 325.6 U/mL, respectively, which were significantly lower than those in the unresectable group (19.1 ± 69.2 ng/mL, 48.8 ± 58.7 U/mL, and 730.1 ± 527.5 U/mL, respectively). According to the results of the ROC curve analysis, the optimal cut-offs for determining the resectability of cholangiocarcinoma were as follows (Figure 1). When CA19-9 was 1064.1 U/mL, it had a sensitivity of 53.2%, specificity of 94%, positive predictive value (PPV) of 80.8%, and negative predictive value (NPV) of 0.73, and the area under the ROC curve (AUC) was 0.73 [95% confidence interval (CI): 0.63–0.82]. When CA125 was 17.8 U/mL, it had a sensitivity of 72.6%, specificity of 78.0%, PPV of 76.7%, and NPV of 74.0%, and the AUC was 0.81 (95% CI: 0.72–0.89). When CEA was 17.8 U/mL, it had a sensitivity of 79.0%, specificity of 48.0%, PPV of 75.5%, and NPV of 53.0%, and the AUC was 0.66 (95% CI: 0.56–0.76).
Figure 1.
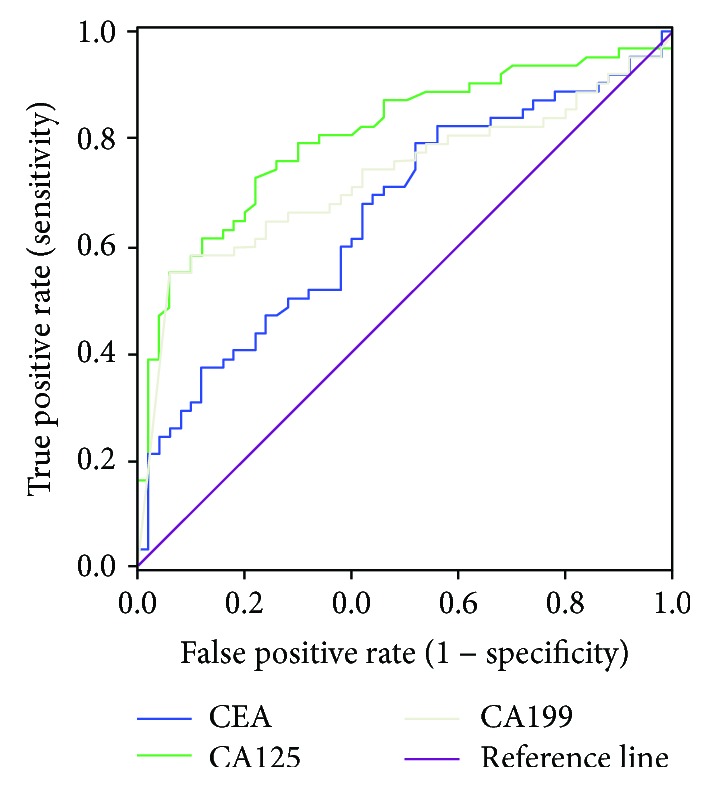
ROC curves for serum CEA, CA125, and CA19-9 levels in the determination of cholangiocarcinoma resectability. The AUC is 0.66 for CEA, 0.81 for CA125, and 0.73 for CA19-9.
By logistic regression analysis, we found that using the combination of three tumor markers could improve the value of predicting resectability of cholangiocarcinoma (Figure 2). The results of ROC curve analysis showed that when we used the combination of CEA and CA125, the AUC was 0.81 (95% CI: 0.89–0.73); when we used the combination of CEA and CA19-9, the AUC was 0.75 (95% CI: 0.84–0.66); when we used the combination of CA125 and CA19-9, the AUC was 0.74 (95% CI: 0.83–0.64); and when we used the combination of CEA, CA125, and CA19-9, the AUC was 0.87 (95% CI: 0.92–0.78). (P < 0.05).
Figure 2.
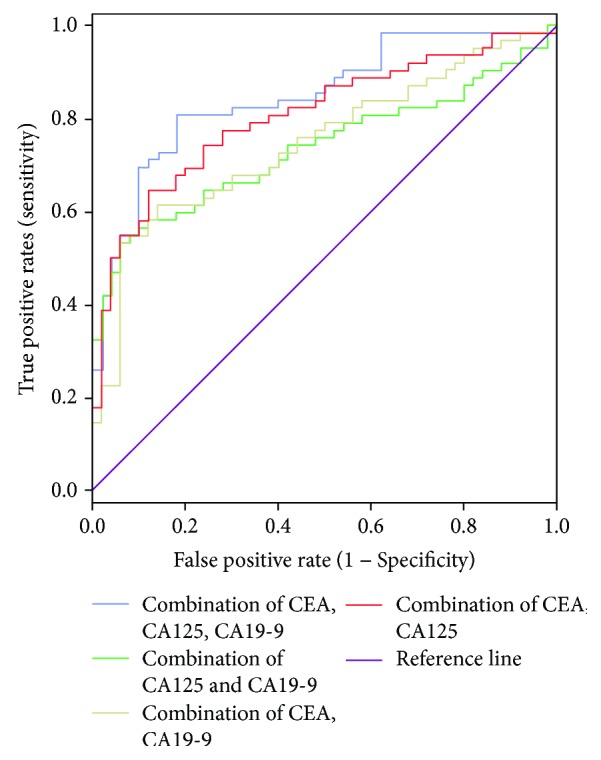
ROC curves for the combination of serum CEA, CA125, and CA19-9 levels in the determination of cholangiocarcinoma resectability. For the combination of CEA and CA125, the AUC is 0.81; for the combination of CEA and CA19-9, the AUC is 0.75; for the combination of CEA, CA125 and CA19-9, the AUC is 0.74; and for the combination of CEA, CA125, and CA19-9, the AUC is 0.87.
3.3. Value of Serum Total Bilirubin in Determining Cholangiocarcinoma Resectability
ROC curve analysis showed that the AUC was 0.54 (95% CI: 0.43–0.65), suggesting that serum total bilirubin had an extremely low accuracy in predicting the resectability of cholangiocarcinoma (Figure 3).
Figure 3.
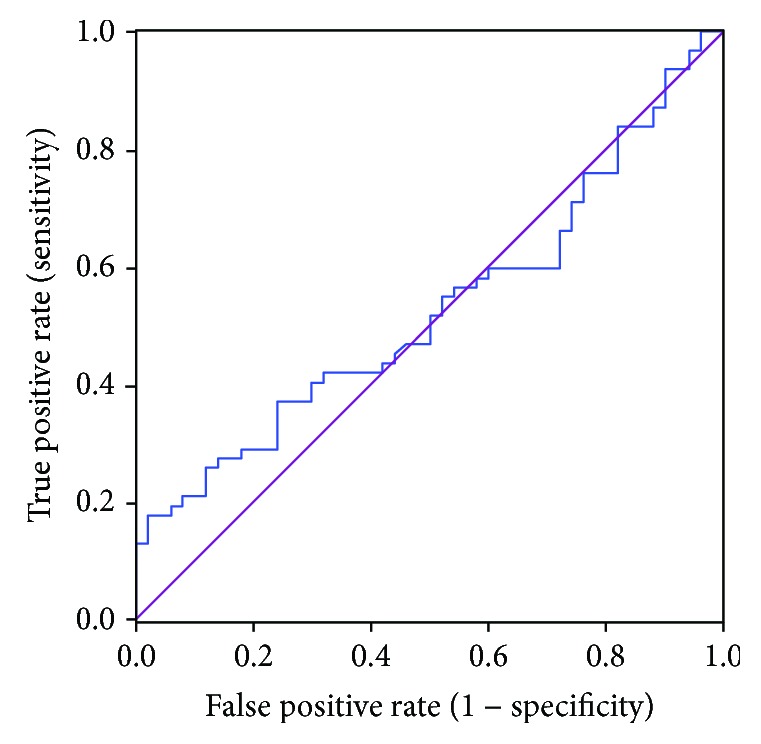
The ROC curve of serum total bilirubin level in the determination of cholangiocarcinoma resectability. The blue line is the total bilirubin, and the purple line is the reference line. The AUC of total bilirubin is 0.54.
4. Discussion
Surgery remains the treatment of choice for cholangiocarcinoma [25, 26]. Radical resection can be achieved if the patient's general condition can tolerate the operation, and there is no distant metastasis [27–30]. Despite rapid advances in surgical techniques and postoperative management, the overall resection rate of cholangiocarcinoma remains low [1, 3, 31], and the incidence of postoperative fatal complications is still high [26, 32, 33]. Therefore, accurate and reliable determination of the resectability of cholangiocarcinoma before surgery is important. CT is the most common examination for determining the resectability of cholangiocarcinoma [34, 35]. However, it is often unable to detect occult metastatic lesions in the liver or abdominal cavity and may miss vascular invasion, resulting in unnecessary surgical trauma and waste of medical resources. Endoscopic ultrasonography and laparoscopy can also be used to determine the resectability before surgery, but they are time-consuming, invasive, and expensive. CEA, CA125, and CA19-9 are the most commonly used tumor markers for preoperative diagnosis and postoperative prognosis prediction of cholangiocarcinoma [36, 37]. According to Juntermanns et al. [38], serum CEA and CA19-9 levels are correlated with the stage of cholangiocarcinoma, and patients with higher preoperative CEA and CA19-9 levels tend to have poorer survival and prognosis. Hatzaras et al. [39] reported that a high preoperative serum CA19-9 level often suggests a low survival rate in patients with bile system cancer. However, little is known about the effects of these tumor markers on the resectability of cholangiocarcinoma.
In the present study, we analyzed the resectability of cholangiocarcinoma based on ROC curve analysis. We found that serum CA19-9 is one of the predictors of cholangiocarcinoma, with an AUC of 0.73 and an optimal cut-off of 1064.1 U/mL. Unlike many other previous studies, our results did not rule out the effect of high bilirubin level on CA19-9, mainly for the following two reasons: cholangiocarcinoma is characterized clinically by its insidious onset, and jaundice, as one of the early symptoms of cholangiocarcinoma, is highly suggestive for this disease in most patients [40–42]. Most of our patients presented with jaundice as the first symptom. Therefore, predicting the surgical resectability by analyzing the serum CA19-9 level in patients with jaundice is particularly significant. In contrast, it is believed that serum CA19-9 is mainly affected by tumor severity and serum bilirubin level [37, 43, 44]; however, it is not possible to completely rule out the effect of serum bilirubin and merely analyze the relationship between serum CA19-9 elevation and surgical resection rate in the statistical analysis. The results of our current analysis were more representative of resectability of cholangiocarcinoma. We also analyzed the relationship between bilirubin level and surgical resection rate of cholangiocarcinoma and found that its predictive value was extremely low, suggesting the feasibility of analyzing serum CA19-9 in patients with jaundice. Since CA19-9 is valuable in predicting the resectability of cholangiocarcinoma [45, 46], it can be used as a supplementary tool for preoperative imaging and for comprehensive evaluation of the success rate of an operation, so as to avoid unnecessary surgery.
Notably, serum CA 125 level had a higher correlation with the resectability of cholangiocarcinoma than CA19-9, which may be explained by the fact that CA125 is less affected by bilirubin. In our study, the AUC of CA125 was 0.81 and the optimal cut-off was 17.8 U/mL. Therefore, it is necessary to measure serum CA125 before surgery, together with CA19-9 as an auxiliary marker, to compensate for the defect of preoperative imaging in predicting resectability and to better guide the treatment.
We also analyzed the relationship between serum CEA and the resectability of cholangiocarcinoma. For CEA, the AUC was 0.66, and the optimal cut-off was 2.6 g/mL; the sensitivity of CEA in predicting the resectability was 79.0%, along with a specificity of 48.0%, PPV of 75.5%, and NPV of 53.0%. However, CEA is a broad-spectrum tumor marker and cannot be used as a specific marker for the diagnosis of a malignancy [47, 48]. Therefore, the value of serum CEA in predicting the resectability of cholangiocarcinoma was lower than that of CA19-9 and CA125.
Generally it is not accurate to predict the resectability of cholangiocarcinoma using a single marker [6, 49]. The value of combining three tumor markers in evaluating resectability of cholangiocarcinoma is higher than that of two. Therefore, it is clinically significant for the preoperative detection of tumor markers in patients with cholangiocarcinoma.
On the other hand, tumor markers are supplement preoperative imaging [50]. Comprehensive analysis of clinical manifestations, preoperative imaging findings, and other prognostic factors (including tumor size) can better assess the resectability of cholangiocarcinoma and provide feasible, appropriate, and reasonable treatment for patients [51].
We also apply this combination to clinical practice. Figure 4 shows one case of distal cholangiocarcinoma. It was difficult for us to know whether the cancer infiltrated the surrounding tissue by preoperative imaging. However, the CA19-9, CA125, and CEA of the patient were lower than the cut-off point in our study. Then radical resection was performed successfully with a negative postoperative pathological margin. Figures 5 and 6 show one case of hilar cholangiocarcinoma and one case of intrahepatic cholangiocarcinoma. It was judged resectable according to our prediction, and the radical resection was performed successfully.
Figure 4.
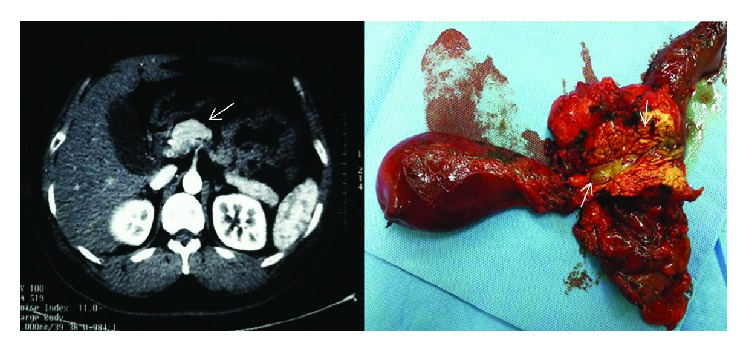
Findings of computed tomography: enhanced computed tomography (CT) revealed a marked high-density area at the distal bile duct (arrowhead). Macroscopic findings: opened bile duct of the resected specimen (arrowhead). Gross appearance of the cut surface of the resected ampulla shows a gray-white tumor measuring 16 mm × 5 mm × 8 mm (arrowhead).
Figure 5.
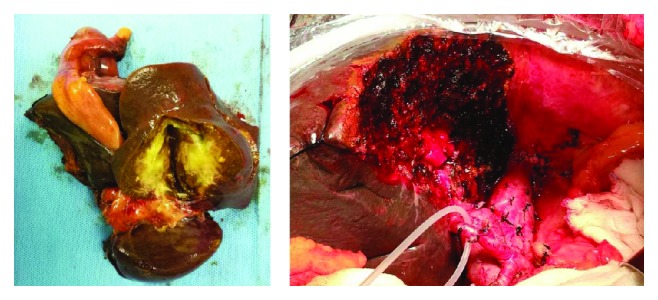
Macroscopic findings: gross appearance of the cut surface of the resected liver shows a gray-white tumor measuring 16 mm × 5 mm × 8 mm.
Figure 6.
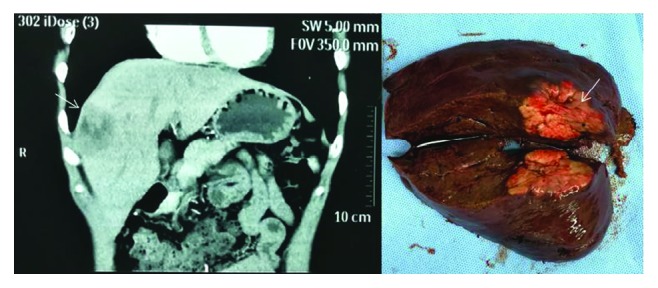
Findings of computed tomography: sagittal plane of computed tomography shows the low-density area (arrowhead) at the right anterior lobe of the liver. Macroscopic findings: gross appearance of the resected liver shows a gray-white tumor measuring 34 mm × 32 mm × 19 mm (arrowhead).
In addition, our retrospective analysis also had limitations in the study design. First, most of the selected patients were diagnosed late and had accompanying hyperbilirubinemia, so it was not possible to accurately analyze the relationship between serum CA19-9 level and resectability of cholangiocarcinoma. Second, our study included patients with jaundice and the results need to be further validated in more comprehensive multicenter studies.
5. Conclusions
In conclusion, preoperative serum CEA, CA19-9, and CA125 levels are useful in predicting the resectability of cholangiocarcinoma and may become supplementary diagnostic indicators for evaluating the resectability of cholangiocarcinoma in the future.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (61572227 and 81170426).
Contributor Information
Yunfu Cui, Email: yfcui777@hotmail.com.
Zhidong Wang, Email: wzd98y2@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Zhidong Wang and Yunfu Cui can be considered co-corresponding authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Tianyi Fang, Hao Wang, Zhidong Wang, and Yunfu Cui contributed equally to this paper. Tianyi Fang conceived, designed, and performed the experiments, analyzed the data, and wrote the paper. Hao Wang conceived and designed the experiments. Yufu Wang and Xuan Lin collected the data. Zhidong Wang and Yunfu Cui supervised the study and gave administrative support for this article.
References
- 1.Rizvi S., Khan S. A., Hallemeier C. L., Kelley R. K., Gores G. J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology. 2017;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Hara S. P., Karlsen T. H., LaRusso N. F. Cholangiocytes and the environment in primary sclerosing cholangitis: where is the link? Gut. 2017;66(11):1873–1877. doi: 10.1136/gutjnl-2017-314249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valle J. W., Lamarca A., Goyal L., Barriuso J., Zhu A. X. New horizons for precision medicine in biliary tract cancers. Cancer Discovery. 2017;7(9):943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgewater J., Galle P. R., Khan S. A., et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. Journal of Hepatology. 2014;60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Bagante F., Tran T., Spolverato G., et al. Perihilar cholangiocarcinoma: number of nodes examined and optimal lymph node prognostic scheme. Journal of the American College of Surgeons. 2016;222(5):750–759.e2. doi: 10.1016/j.jamcollsurg.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird N., Elmasry M., Jones R., et al. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. The British Journal of Surgery. 2017;104(4):418–425. doi: 10.1002/bjs.10399. [DOI] [PubMed] [Google Scholar]
- 7.Maeta T., Ebata T., Hayashi E., et al. Pancreatoduodenectomy with portal vein resection for distal cholangiocarcinoma. The British Journal of Surgery. 2017;104(11):1549–1557. doi: 10.1002/bjs.10596. [DOI] [PubMed] [Google Scholar]
- 8.Chaisaingmongkol J., Budhu A., Dang H., et al. Common molecular subtypes among asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell. 2017;32(1):57–70.e3. doi: 10.1016/j.ccell.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jusakul A., Cutcutache I., Yong C. H., et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discovery. 2017;7(10):1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvaro D. The challenge of cholangiocarcinoma diagnosis: the turning point is in extracellular vesicles? Hepatology. 2017;66(4):1029–1031. doi: 10.1002/hep.29314. [DOI] [PubMed] [Google Scholar]
- 11.Lewis H. L., Rahnemai-Azar A. A., Dillhoff M., Schmidt C. R., Pawlik T. M. Current management of perihilar cholangiocarcinoma and future perspectives. Chirurgia. 2017;112(3):193–207. doi: 10.21614/chirurgia.112.3.193. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto M., Yamashita Y. I., Imai K., et al. Predictors of cure of intrahepatic cholangiocarcinoma after hepatic resection. Anticancer Research. 2017;37(12):6971–6975. doi: 10.21873/anticanres.12164. [DOI] [PubMed] [Google Scholar]
- 13.Wang K., Zhang H., Xia Y., Liu J., Shen F. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surgery and cholangiocarcinoma. 2017;6(2):79–90. doi: 10.21037/hbsn.2017.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosokawa I., Shimizu H., Yoshidome H., et al. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Annals of Surgery. 2014;259(6):1178–1185. doi: 10.1097/SLA.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi E., Fukasawa M., Sato T., et al. Biliary drainage strategy of unresectable malignant hilar strictures by computed tomography volumetry. World Journal of Gastroenterology. 2015;21(16):4946–4953. doi: 10.3748/wjg.v21.i16.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh A., Mann H. S., Thukral C. L., Singh N. R. Diagnostic accuracy of MRCP as compared to ultrasound/CT in patients with obstructive jaundice. Journal of Clinical and Diagnostic Research. 2014;8(3):103–107. doi: 10.7860/JCDR/2014/8149.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolscheid-Pommerich R. C., Manekeller S., Walgenbach-Brünagel G., et al. Clinical performance of CEA, CA19-9, CA15-3, CA125 and AFP in gastrointestinal cancer using LOCI™-based assays. Anticancer Research. 2017;37(1):353–360. doi: 10.21873/anticanres.11329. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita S., Passot G., Aloia T. A., et al. Prognostic value of carbohydrate antigen 19-9 in patients undergoing resection of biliary tract cancer. The British Journal of Surgery. 2017;104(3):267–277. doi: 10.1002/bjs.10415. [DOI] [PubMed] [Google Scholar]
- 19.Verberne C. J., Zhan Z., van den Heuvel E. R., et al. Survival analysis of the CEAwatch multicentre clustered randomized trial. The British Journal of Surgery. 2017;104(8):1069–1077. doi: 10.1002/bjs.10535. [DOI] [PubMed] [Google Scholar]
- 20.Felder M., Kapur A., Gonzalez-Bosquet J., et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Molecular Cancer. 2014;13(1):p. 129. doi: 10.1186/1476-4598-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartella I., Dufour J. F. Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. Journal of Gastrointestinal and Liver Diseases. 2015;24(4):481–489. doi: 10.15403/jgld.2014.1121.244.chl. [DOI] [PubMed] [Google Scholar]
- 22.Bergquist J. R., Puig C. A., Shubert C. R., et al. Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a National Cancer Database Study. Journal of the American College of Surgeons. 2016;223(1):52–65. doi: 10.1016/j.jamcollsurg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Gidwani K., Huhtinen K., Kekki H., et al. A nanoparticle-lectin immunoassay improves discrimination of serum CA125 from malignant and benign sources. Clinical Chemistry. 2016;62(10):1390–1400. doi: 10.1373/clinchem.2016.257691. [DOI] [PubMed] [Google Scholar]
- 24.Feng F., Tian Y., Xu G., et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17(1):p. 737. doi: 10.1186/s12885-017-3738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X.-F., Beal E. W., Bagante F., et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. The British Journal of Surgery. 2018;105(7):848–856. doi: 10.1002/bjs.10676. [DOI] [PubMed] [Google Scholar]
- 26.Doussot A., Lim C., Gómez-Gavara C., et al. Multicentre study of the impact of morbidity on long-term survival following hepatectomy for intrahepatic cholangiocarcinoma. The British Journal of Surgery. 2016;103(13):1887–1894. doi: 10.1002/bjs.10296. [DOI] [PubMed] [Google Scholar]
- 27.Yoh T., Hatano E., Seo S., et al. Long-term survival of recurrent intrahepatic cholangiocarcinoma: the impact and selection of repeat surgery. World Journal of Surgery. 2018;42(6):1848–1856. doi: 10.1007/s00268-017-4387-7. [DOI] [PubMed] [Google Scholar]
- 28.Komaya K., Ebata T., Shirai K., et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. The British Journal of Surgery. 2017;104(4):426–433. doi: 10.1002/bjs.10452. [DOI] [PubMed] [Google Scholar]
- 29.Ebata T., Mizuno T., Yokoyama Y., Igami T., Sugawara G., Nagino M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. The British Journal of Surgery. 2017;105(7):829–838. doi: 10.1002/bjs.10556. [DOI] [PubMed] [Google Scholar]
- 30.Ethun C. G., Lopez-Aguiar A. G., Pawlik T. M., et al. Distal cholangiocarcinoma and pancreas adenocarcinoma: are they really the same disease? A 13-institution study from the US extrahepatic biliary malignancy consortium and the central pancreas consortium. Journal of the American College of Surgeons. 2017;224(4):406–413. doi: 10.1016/j.jamcollsurg.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atanasov G., Schierle K., Hau H. M., et al. Prognostic significance of tumor necrosis in hilar cholangiocarcinoma. Annals of Surgical Oncology. 2017;24(2):518–525. doi: 10.1245/s10434-016-5472-0. [DOI] [PubMed] [Google Scholar]
- 32.Farid S. G., White A., Khan N., Toogood G. J., Prasad K. R., Lodge J. P. A. Clinical outcomes of left hepatic trisectionectomy for hepatobiliary malignancy. The British Journal of Surgery. 2016;103(3):249–256. doi: 10.1002/bjs.10059. [DOI] [PubMed] [Google Scholar]
- 33.Takagi T., Yokoyama Y., Kokuryo T., Yamaguchi J., Nagino M. Liver regeneration following experimental major hepatectomy with choledochojejunostomy. The British Journal of Surgery. 2015;102(11):1410–1417. doi: 10.1002/bjs.9908. [DOI] [PubMed] [Google Scholar]
- 34.Kim J. H., Yoon H. K., Ko G. Y., et al. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255(1):270–277. doi: 10.1148/radiol.09091076. [DOI] [PubMed] [Google Scholar]
- 35.Olthof P. B., Wiggers J. K., Groot Koerkamp B., et al. Postoperative liver failure risk score: identifying patients with resectable perihilar cholangiocarcinoma who can benefit from portal vein embolization. Journal of the American College of Surgeons. 2017;225(3):387–394. doi: 10.1016/j.jamcollsurg.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Li D. J., Chen J., et al. Application of joint detection of AFP, CA19-9, CA125 and CEA in identification and diagnosis of cholangiocarcinoma. Asian Pacific Journal of Cancer Prevention. 2015;16(8):3451–3455. doi: 10.7314/APJCP.2015.16.8.3451. [DOI] [PubMed] [Google Scholar]
- 37.Hu H. J., Mao H., Tan Y. Q., et al. Clinical value of preoperative serum CA 19-9 and CA 125 levels in predicting the resectability of hilar cholangiocarcinoma. Springerplus. 2016;5(1):p. 551. doi: 10.1186/s40064-016-2181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juntermanns B., Radunz S., Heuer M., et al. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. European Journal of Medical Research. 2010;15(8):357–361. doi: 10.1186/2047-783X-15-8-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatzaras I., Schmidt C., Muscarella P., Melvin W. S., Ellison E. C., Bloomston M. Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB: The Official Journal of the International Hepato Pancreato Biliary Association. 2010;12(2):134–138. doi: 10.1111/j.1477-2574.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banales J. M., Cardinale V., Carpino G., et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nature Reviews. Gastroenterology & Hepatology. 2016;13(5):261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 41.Sapisochin G., Facciuto M., Rubbia-Brandt L., et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64(4):1178–1188. doi: 10.1002/hep.28744. [DOI] [PubMed] [Google Scholar]
- 42.Tsukahara T., Ebata T., Shimoyama Y., et al. Residual carcinoma in situ at the ductal stump has a negative survival effect: an analysis of early-stage cholangiocarcinomas. Annals of Surgery. 2017;266(1):126–132. doi: 10.1097/SLA.0000000000001944. [DOI] [PubMed] [Google Scholar]
- 43.Bangarulingam S. Y., Bjornsson E., Enders F., et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51(1):174–180. doi: 10.1002/hep.23277. [DOI] [PubMed] [Google Scholar]
- 44.Lian P. L., Chang Y., Xu X. C., Zhao Z., Wang X. Q., Xu K. S. Pancreaticoduodenectomy for duodenal papilla carcinoma: a single-centre 9-year retrospective study of 112 patients with long-term follow-up. World Journal of Gastroenterology. 2017;23(30):5579–5588. doi: 10.3748/wjg.v23.i30.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizvi S., Gores G. J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X. W., Huang Y., Chen L. D., et al. Potential diagnostic performance of contrast-enhanced ultrasound and tumor markers in differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and cholangiocarcinoma. Journal of Medical Ultrasonics. 2018;45(2):231–241. doi: 10.1007/s10396-017-0834-1. [DOI] [PubMed] [Google Scholar]
- 47.Carr R. A., Yip-Schneider M. T., Dolejs S., et al. Pancreatic cyst fluid vascular endothelial growth factor a and carcinoembryonic antigen: a highly accurate test for the diagnosis of serous cystic neoplasm. Journal of the American College of Surgeons. 2017;225(1):93–100. doi: 10.1016/j.jamcollsurg.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He M. Q., Wang K., Wang W. J., Yu Y. L., Wang J. H. Smart DNA machine for carcinoembryonic antigen detection by exonuclease III-assisted target recycling and DNA walker cascade amplification. Analytical Chemistry. 2017;89(17):9292–9298. doi: 10.1021/acs.analchem.7b02073. [DOI] [PubMed] [Google Scholar]
- 49.Le Roy B., Gelli M., Pittau G., et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. The British Journal of Surgery. 2017;105(7):839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 50.Shafaghi A., Mansour- Ghanaei F., Joukar F., Nabavi F., Mansour- Ghanaei A., Esrafilian Soltani A. Stage association of preoperative serum carcinoembryonic antigen with gastric adenocarcinoma in Iranian patients. Asian Pacific Journal of Cancer Prevention. 2017;18(10):2669–2672. doi: 10.22034/APJCP.2017.18.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada M., Ebata T., Yokoyama Y., et al. Pulmonary metastasis after resection of cholangiocarcinoma: incidence, resectability, and survival. World Journal of Surgery. 2017;41(6):1550–1557. doi: 10.1007/s00268-017-3877-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


