Abstract
Galium verum L. (G. verum, lady's bedstraw) is a perennial herbaceous plant, belonging to the Rubiaceae family. It has been widely used throughout history due to multiple therapeutic properties. However, the effects of this plant species on functional recovery of the heart after ischemia have still not been fully clarified. Therefore, the aim of our study was to examine the effects of methanol extract of G. verum on myocardial ischemia/reperfusion (I/R) injury in spontaneously hypertensive rats (SHR), with a special emphasis on the role of oxidative stress. Rats involved in the research were divided randomly into two groups: control (spontaneously hypertensive rats (SHR)) and G. verum group, including SHR rats treated with the G. verum extract (500 mg/kg body weight per os) for 4 weeks. At the end of the treatment, in vivo cardiac function was assessed by echocardiography. Rats were sacrificed and blood samples were taken for spectrophotometric determination of systemic redox state. Hearts from all rats were isolated and retrogradely perfused according to the Langendorff technique. After a stabilization period, hearts were subjected to 20-minute ischemia, followed by 30-minute reperfusion. Levels of prooxidants were spectrophotometrically measured in coronary venous effluent, while antioxidant enzymes activity was assessed in heart tissue. Cell morphology was evaluated by hematoxylin and eosin (HE) staining. 4-week treatment with G. verum extract alleviated left ventricular hypertrophy and considerably improved in vivo cardiac function. Furthermore, G. verum extract preserved cardiac contractility, systolic function, and coronary vasodilatory response after ischemia. Moreover, it alleviated I/R-induced structural damage of the heart. Additionally, G. verum extract led to a drop in the generation of most of the measured prooxidants, thus mitigating cardiac oxidative damage. Promising potential of G. verum in the present study may be a basis for further researches which would fully clarify the mechanisms through which this plant species triggers cardioprotection.
1. Introduction
Acute myocardial infarction (AMI) is one of the most common causes of disability and mortality worldwide [1]. Although timely restoration of blood flow to an ischemic heart is essential for limiting the infarct size, it can paradoxically exacerbate tissue damage. This phenomenon is known as ischemia/reperfusion (I/R) injury and occurs in several forms such as reperfusion-induced arrhythmias, myocardial stunning, microvascular obstruction, and reperfusion-induced cardiomyocyte death [2]. The exact mechanisms underlying progression of I/R injury have been under intense investigation for more than four decades with the aim to open the way to novel effective strategies for myocardial salvage [3]. Factors that have been proven to mostly contribute to myocardial dysfunction are increased generation of prooxidants, pronounced inflammatory response, and intracellular and mitochondrial Ca2+ overload [3, 4]. Presence of hypertension, as one of the most common risk factors for cardiovascular diseases, may worsen I/R outcome due to high myocardial load/mechanical stress [5].
Ischemic preconditioning is a successful maneuver for reaching cardioprotection by exposing the heart to brief episodes of ischemia and reperfusion prior to prolonged ischemia. Due to its limited clinical application, recent researches have focused its attention on agents that might be pharmacological mimetics of ischemic preconditioning [4]. Growing evidence suggest benefits of dietary intake of medicinal plants rich in polyphenols in lowering the incidence of coronary diseases [6, 7]. Polyphenols are biologically active constituents found in vegetables, fruits, wines, etc., with great potential to attenuate the deleterious impact of I/R via antioxidant and anti-inflammatory activities and preservation of nitric oxide [7].
Galium verum (G. verum, lady's bedstraw) is a perennial herbaceous plant, belonging to the Rubiaceae family and has been widely recognized throughout history due to its multiple therapeutic properties [8]. It has been used as a sedative, an anticancer agent, in the treatment of gout and epilepsy; nevertheless, effects of this plant species on cardiac function have not been clarified yet [9]. Phytochemical investigations revealed the presence of iridoid glycosides, phenolic compounds (such as chlorogenic and caffeic acids, hyperoside), anthraquinones, and triterpenes in G. verum extracts [8, 9]. Therefore, we hypothesized that G. verum extract might be involved in the alleviation of harmful effects of I/R injury on functional and morphological properties of the heart.
Regarding above-mentioned data the aim of our study was to assess the effects of methanol extract of G. verum on myocardial I/R injury in spontaneously hypertensive rats (SHR), with a special emphasis on the role of oxidative stress.
2. Materials and Methods
2.1. Ethical Approval
This investigation was carried out in the laboratory for cardiovascular physiology of the Faculty of Medical Sciences, University of Kragujevac, Serbia. The study protocol was approved by the Ethical Committee for the welfare of experimental animals of the Faculty of Medical Sciences, University of Kragujevac, Serbia. All experiments were performed according to the EU Directive for the welfare of laboratory animals (86/609/EEC) and the principles of Good Laboratory Practice (GLP).
2.2. Plant Material and Extract Preparation
The whole plant G. verum was collected on July 5th, 2017 in the village Dobroselica on the southern cliff of Mt. Zlatibor (GPS coordinates: 43°42′59.99 ″ N and 19° 41′59.99 ″ E). Identification and classification of the plant material were performed at the Department of Biology, Faculty of Natural Sciences, University of Kragujevac and at the Institute of Botany and Botanical garden “Jevremovac”, University of Belgrade. A voucher specimen (number 17417) was kept in a herbarium of the Institute of Botany and Botanical garden “Jevremovac”. The collected material was dried under the shade and powdered (sieve 0.75). The methanol extract was prepared to extract 100 g of the aerial part of the plant with 500 ml of methanol by heat reflux extraction [10]. The mixture was filtered through a filter paper (Whatman, No. 1), and the dry extract was obtained after removing the solvent by evaporation under reduced pressure (RV05 basic IKA, Germany). Afterwards, the residue (16.03 g) was stored in a dark glass bottle at +4°C until use. Once daily just before administration to experimental animals, extract was dissolved in the tap water.
2.3. Animals
Twenty spontaneously hypertensive rats (males, eight weeks old, body weight 150 ± 30 g) were included in the study. They consumed commercial rat food (20% protein rat food, Veterinary Institute Subotica, Serbia) ad libitum and were housed at a temperature of 22 ± 2°C, with 12 hours of automatic illumination daily. The rats were divided into two groups as follows:
(1) Control Group. Rats who drank only tap water
(2) G. verum Group. Rats who drank tap water containing 500 mg/kg of methanol extract of G. verum
In order to provide a precise amount of drug for every animal, rats were kept in separate cages (one animal per cage). Considering that animals drink approximately 50 ml of water per day, we dissolved individual dose of extract adjusted to body weight (500 mg/kg) in this volume of water. The same procedure was repeated every day of the experimental protocol.
2.4. Assessment of In Vivo Cardiac Function
Transthoracic echocardiograms were performed after accomplishing drinking protocol. A mixture of ketamine 50 mg/kg (100 mg/mL; Ketaset, Fort Dodge, IA) and xylazine 10 mg/kg (100 mg/mL; AnaSed, Lloyd Laboratories, Shenandoah, IA) intraperitoneally was used as anesthesia. Echocardiograms were performed using a Hewlett-Packard Sonos 5500 (Andover, Massachusetts) sector scanner equipped with a 15.0 MHz phased array transducer as previously described [11]. From the parasternal long-axis view in a 2-dimensional mode, M-mode cursor was positioned perpendicularly to the interventricular septum and posterior wall of the left ventricle (LV) at the level of the papillary muscles and M-mode images were obtained. Interventricular septal wall thickness at end diastole (IVSd), LV internal dimension at end diastole (LVIDd), LV posterior wall thickness at end diastole (LVPWd), interventricular septal wall thickness at end systole (IVSs), LV internal diameter at end systole (LVIDs), and LV posterior wall thickness at end systole (LVPWs) were recorded with M-mode. Fractional shortening percentage (FS%) was calculated from the M-mode LV diameters using the equation: [(LVEDd − LVESd)/LVEDd] × 100%.
2.5. Preparation of Isolated Rat Hearts
Following 4 weeks of G. verum extract treatment, after short-term narcosis induced by intraperitoneal application of ketamine (10 mg/kg) and xylazine (5 mg/kg) and premedication with heparin as an anticoagulant, animals were sacrificed by decapitation. Then the chest was opened via midline thoracotomy, the hearts were immediately removed and immersed in cold saline, and the aortas were cannulated and retrogradely perfused under a constant perfusion pressure (CPP) of 70 cmH2O according to the Langendorff technique. The composition of Krebs–Henseleit buffer used for retrograde perfusion was as follows (mmol/L): NaCl 118, KCl 4.7, CaCl2 × 2H2O2.5, MgSO4 × 7H2O 1.7, NaHCO3 25, KH2PO4 1.2, glucose 11, pyruvate 2, equilibrated with 95% O2 plus 5% CO2, and warmed to 37°C (pH 7.4).
2.6. Assessment of Ex Vivo Cardiac Function
After placing the sensor (transducer BS473-0184, Experimetria Ltd., Budapest, Hungary) in the left ventricle, the following parameters of myocardial function have been measured during stabilization and during reperfusion: maximum rate of pressure development in the left ventricle (dp/dt max), minimum rate of pressure development in the left ventricle (dp/dt min), systolic left ventricular pressure (SLVP), diastolic left ventricular pressure (DLVP), and heart rate (HR). Coronary flow (CF) was measured flowmetrically.
At first, the hearts underwent a 30-minute perfusion at CPP of 70 cmH2O in order to achieve a stable rhythm. After a stabilization period, the hearts were exposed to global ischemia (perfusion was totally stopped) for 20 minutes, followed by 30 minutes of reperfusion. All cardiodynamic parameters and coronary flow were measured in intervals of 5 minutes during the period of reperfusion (30 minutes) (RP1-RP7).
2.7. Biochemical Analysis
After sacrificing, blood samples for biochemical analysis were collected from a jugular vein in order to test the systemic redox state. After centrifugation of heparinised venous blood, plasma and erythrocytes were separated. In plasma, the following prooxidants were determined: the levels of superoxide anion radical (O2 −), nitrites (NO2 −), hydrogen peroxide (H2O2), and index of lipid peroxidation (measured as thiobarbituric acid reactive substances (TBARS)). Parameters of the antioxidative defense system such as activities of superoxide dismutase (SOD) and catalase (CAT) and level of reduced glutathione (GSH) were determined in erythrocyte samples. Cardiac oxidative stress markers were determined in coronary venous effluent and heart tissue. After heart stabilization and in the intervals of 5 minutes during the period of reperfusion, coronary venous effluent was collected and used for spectrophotometric determination of levels of TBARS, O2 −, H2O2, and NO2 −. Analyses of the prooxidants were performed using the same methods as when analyzing plasma samples and coronary venous effluent except for NO2 − where the protocol differs. The activity of antioxidant enzymes such as CAT and SOD was determined in heart tissue.
2.7.1. Superoxide Anion Radical Determination (O2 −)
The level of superoxide anion radical (O2 −) was determined using nitro blue tetrazolium (NBT) reaction in TRIS-buffer combined with plasma sample or coronary venous effluent. The measurement was performed at a wavelength of 530 nm. Krebs–Henseleit solvent was used as the blank control for O2 − determination in coronary venous effluent, while distilled water for plasma samples [12].
2.7.2. Hydrogen Peroxide Determination (H2O2)
The protocol for measurement of hydrogen peroxide (H2O2) is based on oxidation of phenol red in the presence of horseradish peroxidase. 200 μl sample with 800 μl PRS (phenol red solution) and 10 μl POD (horseradish peroxidase) were combined (1 : 20). The level of H2O2 was measured at 610 nm. Krebs–Henseleit solvent was used as the blank control for the determination of this parameter in coronary venous effluent, while distilled water was used for plasma samples [13].
2.7.3. Nitrite Determination (NO2 −)
Nitric oxide (NO) decomposes rapidly to form stable metabolite nitrite/nitrate products. Nitrite (NO2 −) was determined as an index of nitric oxide production with Griess reagent. For NO2 − determination in plasma 0.1 ml 3 N PCA (perchloride acid), 0.4 ml 20 mM ethylenediaminetetraacetic acid (EDTA) and 0.2 ml plasma were put on ice for 15 min, then centrifuged for 15 min at 6000 rpm. After pouring off the supernatant, 220 μl K2CO3 was added. Nitrites were measured at 550 nm. Distilled water was used as a blank probe.
In order to determine NO2 − level in coronary venous effluent, 0.5 ml of the perfusate was precipitated with 200 μl of 30% sulfosalicylic acid, mixed for 30 min, and centrifuged at 3000 x g. Equal volumes of the supernatant and Griess reagent were mixed and stabilized for 10 min in the dark, and then the sample was measured spectrophotometrically at a wavelength of 543 nm. The nitrite concentrations were determined using sodium nitrite as the standard [14].
2.7.4. Determination of the Index of Lipid Peroxidation Measured as TBARS
The degree of lipid peroxidation in the sample (plasma and coronary venous effluent) was estimated by measuring of TBARS using 1% TBA (thiobarbituric acid) in 0.05 NaOH, incubated with sample at 100°C for 15 min, and read at 530 nm. TBA extract was obtained by combining 0.8 ml sample and 0.4 ml trichloro acetic acid (TCA); afterwards, the samples were put on ice for 10 min and centrifuged for 15 min at 6000 rpm. For TBARS determination in coronary venous effluent, the Krebs–Henseleit solvent was used as a blank control, while in the case of plasma sample distilled water was used [15].
2.7.5. Determination of Antioxidant Enzymes (CAT, SOD)
Isolated RBCs were washed three times with three volumes of ice-cold 0.9 mmol/l NaCl, and hemolysates containing about 50 g Hb/l [16] were used for the determination of CAT activity [17]. Moreover, hearts from all animals were frozen at -80°C. Additionally, 0.5 section of each tissue was homogenized in 5 ml phosphate buffer (pH 7.4) on ice, and homogenates were centrifuged at 1200 x g for 20 min at 4°C. The resulting supernatants were used for the determination of cardiac activity of CAT and SOD. CAT buffer, prepared sample (hemolysates or heart tissue), and 10 mM H2O2 were used for CAT determination. Detection was performed at 360 nm. The amount of CAT was expressed as U/g tissue and in hemolysate as U/g Hb × 103 [17, 18]. SOD activity was determined by the epinephrine method. Heart tissue or hemolysate was mixed with carbonate buffer, and then epinephrine was added. Detection was performed at 470 nm. The amount of SOD in heart tissue was expressed as U/g tissue and in hemolysate as U/g Hb × 103 [19, 20].
2.7.6. Determination of Reduced Glutathione (GSH)
The level of reduced glutathione (GSH) was determined based on GSH oxidation via 5,5-dithiobis-6,2-nitrobenzoic acid. GSH extract was obtained by combining 0.1 ml 0.1% EDTA, 400 μl hemolysate, and 750 μl precipitation solution (containing 1.67 g metaphosphoric acid, 0.2 g EDTA, 30 g NaCl, and filled with distilled water until 100 ml; the solution is stable for 3 weeks at +4C°). After mixing in the vortex machine and extraction on cold ice (15 min), it was centrifuged at 4000 rpm (10 min). Distilled water was used as a blank probe. The level of GSH was measured at 420 nm. The concentration is expressed as nanomoles per milliliter of red blood cells (RBCs) [21].
2.8. Histological Analysis
In order to evaluate the effects of G. verum on cell morphology after I/R, hematoxylin-eosin staining (HE) was performed for hearts according to the previously reported method [22].
2.9. Statistical Analysis
For statistical analysis of data within and between the control and G. verum group, IBM SPSS Statistics 20.0 for Windows was used. Three measured points were statistically analyzed: the first point was stabilization (S), the second was the first, and the last point of 30-minute reperfusion period (R1 and R7). Values were expressed as mean ± standard deviation (SD). Shapiro–Wilk test was used to check the distribution of data. Additionally, data was analyzed using a one-way analysis of variance (ANOVA) and the post hoc Bonferroni test for multiple comparisons. Values of p < 0.05 were considered to be statistically significant.
3. Results
3.1. Effects of G. verum Extract on Morphological Variables
Values of body weight (BW), heart weight (HW), and HW/BW ratio in both groups of rats are shown in Table 1. The HW/BW ratio was significantly lower in the G. verum group.
Table 1.
Effects of G. verum extract on body and heart weight measurements.
| BW (g) | HW (mg) | HW/BW ratio | |
|---|---|---|---|
| Control | 266.65 ± 14.75 | 1.08 ± 0.07 | 4.95 ± 0.25 |
| G. verum | 235.75 ± 17.78 | 1.02 ± 0.1 | 4.32 ± 0.83∗ |
Values are shown as means ± SD. ∗Statistical significance at the level of p < 0.05 between the control and G. verum group; BW = body weight; HW = heart weight; HW/BW = heart weight to body weight.
3.2. Effects of G. verum Extract on In Vivo Cardiac Function
LVIDd, LVIDs, and LVPWd were significantly decreased after G. verum treatment, while IVSd remained unchanged. Furthermore, midwall fractional shortening was significantly increased in the G. verum group in comparison to control (Table 2).
Table 2.
Effects of G. verum extract on in vivo cardiac function.
| Control | G. verum | |
|---|---|---|
| IVSd (cm) | 0.203 ± 0.011 | 0.208 ± 0.016 |
| LVIDd (cm) | 0.709 ± 0.003 | 0.458 ± 0.013∗ |
| LVPWd (cm) | 0.251 ± 0.013 | 0.176 ± 0.046∗ |
| LVIDs (cm) | 0.432 ± 0.01 | 0.186 ± 0.034∗ |
| LVPWs (cm) | 0.302 ± 0.002 | 0.297 ± 0.03 |
| FS (%) | 39.0 ± 2.34 | 59.3 ± 3.54∗ |
Values are shown as means ± SD. ∗Statistical significance at the level of p < 0.05 between the control and G. verum group; IVSd = end-diastolic interventricular septal thickness; LVIDd = left ventricular internal diameter end diastole; LVPWd = left ventricular end-diastolic posterior wall thickness; LVIDs = left ventricular internal diameter end systole; LVPWs = left ventricular posterior wall thickness; FS = fractional shortening.
3.3. Effects of G. verum Extract on Ex Vivo Cardiac Function
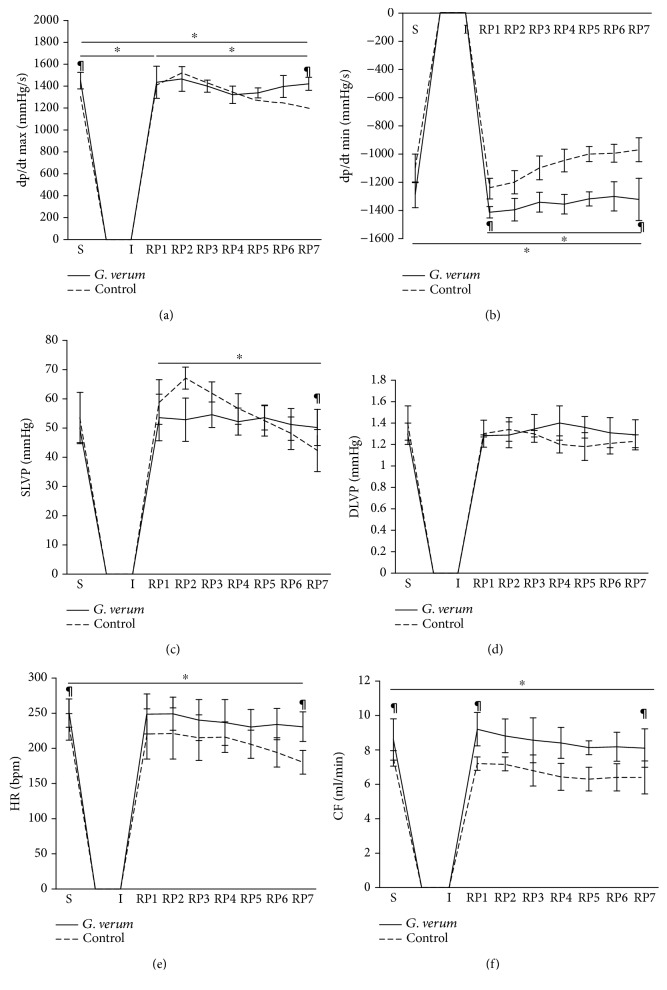
There was a statistically significant increase in the value of the maximum rate of left ventricular pressure development (dp/dt max) at RP1 in comparison to S and RP7 in the control group and a decrease in RP7 in comparison to S point. In the G. verum group, the value of this parameter remained constant during the observed period. Furthermore, the values noticed in S and RP7 points were higher in G. verum than in the control group (Figure 1(a)). There was a drop in the value of the minimum rate of left ventricular pressure development (dp/dt min) at RP7 in comparison to RP1 and S points in the control group. Markedly, higher value of this parameter was noticed in the G. verum group both at RP1 and at RP7 compared to control conditions (Figure 1(b)).
Figure 1.
Effects of G. verum pretreatment on ex vivo cardiac function. (a) Comparison within and between groups in the value of dp/dt max, (b) comparison within and between groups in the value of dp/dt min, (c) comparison within and between groups in the value of SLVP, (d) comparison within and between groups in the value of DLVP, (e) comparison within and between groups in the value of heart rate, and (f) comparison within and between groups in the value of coronary flow. ∗Statistical significance at the level of p < 0.05 within the control group; #statistical significance at the level of p < 0.05 within the G. verum group; ¶statistical significance at the level of p < 0.05 between the control and G. verum group. Data are presented as means ± SD. R1, first minute of reperfusion. R7, last minute of reperfusion.
A significant drop in systolic blood pressure in the left ventricle (SLVP) was found in the control group at RP7 when compared to RP1, while in the G. verum group SLVP value did not vary significantly. In the G. verum extract pretreated group, higher SLVP was observed at RP7 (Figure 1(c)). There was no statistically significant change in the value of diastolic blood pressure in the left ventricle (DLVP) both within and between groups (Figure 1(d)).
In the control group, a drop in heart rate (HR) was noticed at RP7 in comparison to S point. When compared to G. verum and control group, higher HR was noticed at RP7 and S points (Figure 1(e)). Coronary flow (CF) decreased at RP7 compared to S in the control group, and the value of the observed parameter was lower in this group in all points in comparison to the G. verum group (Figure 1(f)).
3.4. Effects of G. verum Extract on Systemic Redox State
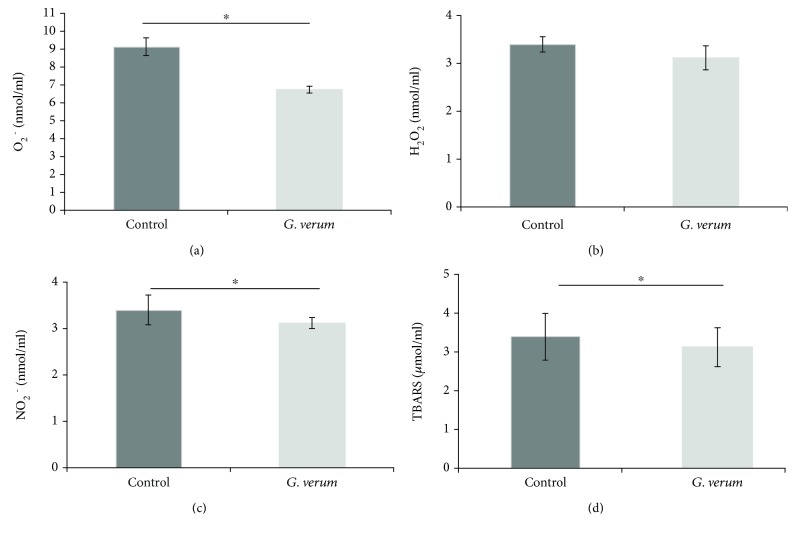
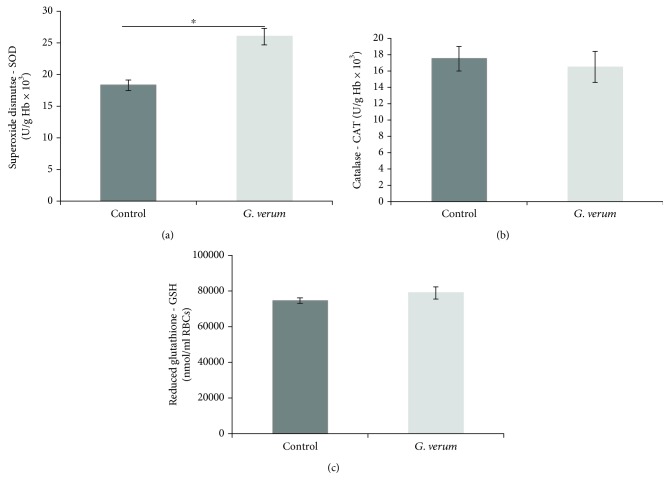
The levels of oxidative stress markers, such as O2 −, NO2 −, and TBARS, were decreased in the G. verum group in comparison to the control group, while there was no difference in the level of H2O2 (Figure 2). Regarding the parameters of antioxidant defense system, there was an increase in SOD activity in the G. verum group, while the activity of CAT and level of GSH were not changed compared to the control group (Figure 3).
Figure 2.
Effects of G. verum pretreatment on the level of prooxidants determined in plasma samples. (a) Comparison between groups in the value of O2 −, (b) comparison between groups in the value of H2O2, (c) comparison between groups in the value of NO2 −, and (d) comparison between groups in the value of TBARS. ∗Statistical significance at the level of p < 0.05 between the control and G. verum group. Data are presented as means ± SE.
Figure 3.
Effects of G. verum pretreatment on parameters of antioxidant defense system determined in erythrocytes samples. (a) Comparison between groups in the activity of SOD, (b) comparison between groups in the activity of CAT, and (c) comparison between groups in the level of GSH. ∗Statistical significance at the level of p < 0.05 between the control and G. verum group. Data are presented as means ± SE.
3.5. Effects of G. verum Extract on Cardiac Redox State
3.5.1. Superoxide Anion Radical (O2 −)
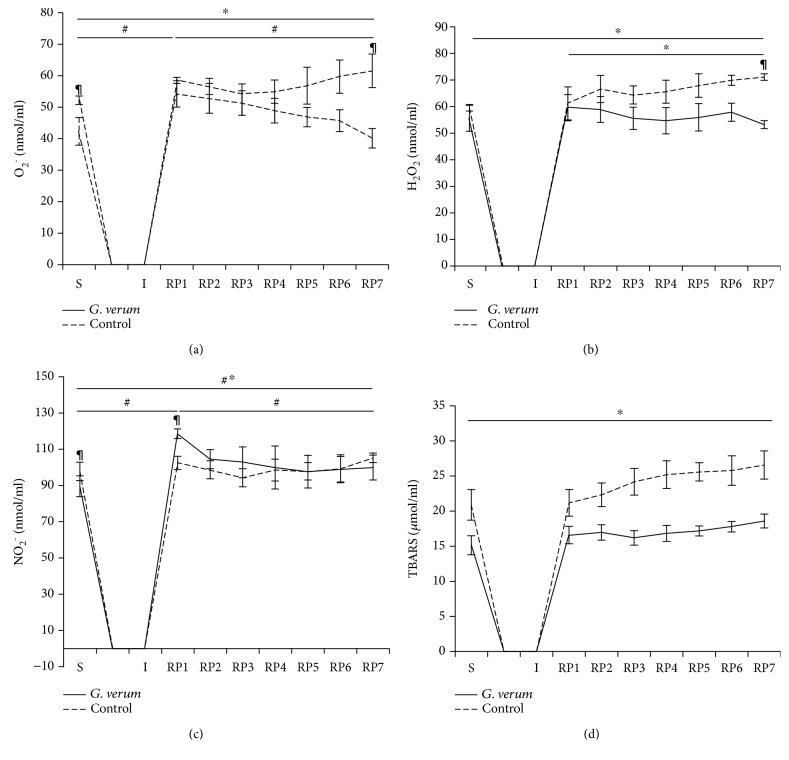
There was a rise in O2 − at RP7 in comparison to S point in the control group. Furthermore, G. verum extract led to an increase in the level of this parameter in RP1 compared to S; however, it significantly decreased at RP7 and reached the values similar from the S point. The levels of O2 − both at S and RP7 moments were significantly higher in the control group (Figure 4(a)).
Figure 4.
Effects of G. verum pretreatment on the level of cardiac prooxidants. (a) Comparison within and between groups in the value of O2 −, (b) comparison within and between groups in the value of H2O2, (c) comparison within and between groups in the value of NO2 −, and (d) comparison within and between groups in the value of TBARS. ∗Statistical significance at the level of p < 0.05 within the control group; #statistical significance at the level of p < 0.05 within the G. verum group; ¶statistical significance at the level of p < 0.05 between the control and G. verum group. Data are presented as means ± SE. R1, first minute of reperfusion. R7, last minute of reperfusion.
3.5.2. Hydrogen Peroxide (H2O2)
A significantly elevated level of H2O2 was noticed in RP7 compared to S and RP1 in the control group, while in the G. verum group this parameter did not change during the time. A pronounced rise in the level of this oxidative stress marker was detected in the control group in comparison to G. verum at RP7 (Figure 4(b)).
3.5.3. Nitrites (NO2 −)
In the control group, there was an increase in the level of NO2 − in RP7 in comparison to S point. On the other hand, in the G. verum group an increase in the level of this parameter was noticed in RP1 compared to S and RP7 and in RP7 compared to the S point. In S and RP1 moments, the level of NO2 − was higher in the group pretreated with G. verum extract (Figure 4(c)).
3.5.4. Index of Lipid Peroxidation Measured as TBARS
The level of TBARS was elevated in RP7 compared to S in the control group. Additionally, the level of this marker in the G. verum group in RP7 was significantly lower than in the control group (Figure 4(d)).
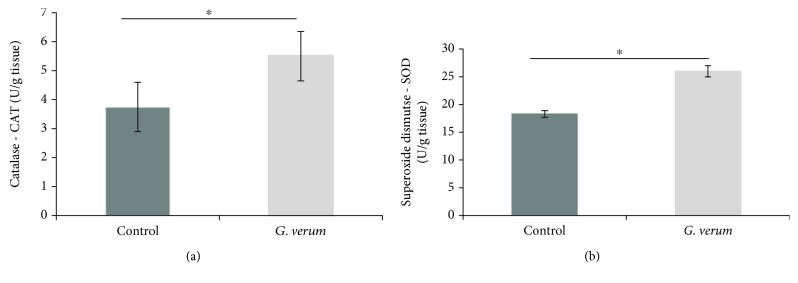
3.5.5. Catalase (CAT) and Superoxide Dismutase (SOD)
The cardiac activity of CAT and SOD was significantly increased in the G. verum group (Figure 5).
Figure 5.
Effects of G. verum pretreatment on cardiac antioxidant enzymes activity. (a) Comparison between groups in the activity of CAT and (b) comparison between groups in the activity of SOD. ∗Statistical significance at the level of p < 0.05 between the control and G. verum group. Data are presented as means ± SE.
3.6. Effects of G. verum Extract on Heart Morphology
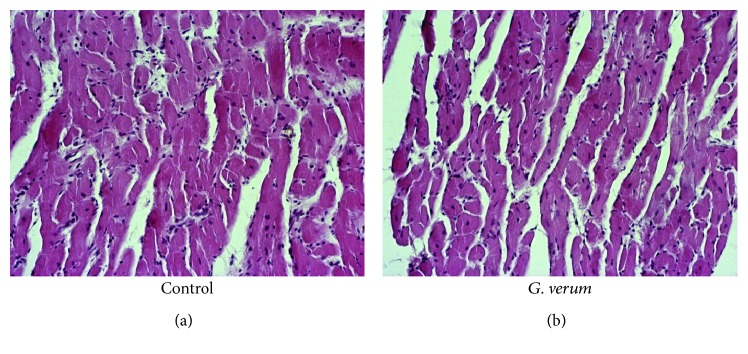
Hypertrophy of the cardiac muscle fibers, edema, necrosis, and degenerative changes were observed in both groups. However, in the control group, degenerative changes were more prominent involving zonal necrosis of higher number of cardiomyocytes, with hypereosinophilia, fragmentation of the fibers, and loss of nucleus. Moreover, focal necrosis of lower number of cardiomyocytes or unicellular was found in the G. verum group (Figure 6).
Figure 6.
Histopathological changes in the myocardium following reperfusion (magnification, ×200). Control group: hypertrophy of the cardiac muscle fibers, edema, necrosis, and zonal necrosis of higher number of cardiomyocytes, with hypereosinophilia, fragmentation of the fibers, and loss of nucleus. G. verum group: hypertrophy of the cardiac muscle fibers, edema, necrosis, focal necrosis of lower number of cardiomyocytes or unicellular, and less prominent degenerative changes.
4. Discussion
It is well established that hypertension may lead to left ventricular hypertrophy development, thus increasing mortality from AMI and vulnerability of myocardium to I/R injury [23, 24]. Advances in understanding the complexity of mechanisms involved in the pathogenesis of myocardial I/R injury points to a central role of oxidative stress. Therefore, growing scientific attention was focused on the modulation of redox homeostasis with plants and plant-derived products rich in natural antioxidants, thus reducing postischemic myocardial dysfunction [6]. Previous investigations have proven the antioxidant activity of G. verum extract in vitro [8, 25]. However, to our best knowledge, there is no data regarding its effects on heart function and potential of G. verum extract to attenuate myocardial damage, and diminish oxidative stress is open to discussion. We hypothesized that G. verum extract treatment might help salvage the ischemic-reperfused heart.
An echocardiographic examination of heart function demonstrated that 4-week G. verum treatment decreased LVIDd, LVIDs, and LVPWd and increased FS compared to the untreated SHR group, thus mitigating left ventricular hypertrophy and considerably improving cardiac function. This is in line with previous findings showing that natural substances belonging to a group of flavonoids might ameliorate endothelial dysfunction and cardiac hypertrophy in the spontaneously hypertensive rats [26, 27]. Additionally, the beneficial effect of isoflavones on overall cardiovascular markers in models of LV dysfunction, involving enhancement of percent FS, has been proven so far [28].
Our results clearly show that 20-minute ex vivo ischemia on Langendorff apparatus impaired myocardial function manifested as a marked reduction in dp/dt max, dp/dt min, SLVP, HR, and CF in the control group. However, in the G. verum group, values of dp/dt max and dp/dt min were unchanged during the reperfusion period, enabling the cardiac muscle to contract and relax without variations in force. In support of this finding, it was found that protective effects on ventricular contractility may be reached by either application of flavonoids alone or plant extracts containing polyphenolics, flavonoids, iridoids, and terpenoids [29, 30]. Moreover, in animals receiving G. verum, systolic function was not only preserved but even improved, as evidenced by an increase in SLVP. G. verum extract consumption prevented I/R-induced decline in HR, so unaltered HR provided enough time for the heart to contract strongly. Additionally, prolonged ischemia led to a coronary vascular dysfunction reflected in reduced CF in the control group. In the G. verum extract pretreated group, coronary flow was slightly but not significantly changing over 30 min recovery period, so at the end of reperfusion it returned to baseline values. This fact suggested that preconditioning with G. verum preserved the coronary vasodilatory response to I/R.
The presence of flavonoids in G. verum extracts was confirmed in previous studies [8, 9]. Striking evidence indicate that flavonoids have the potential to improve postischemic heart functionality [29, 30]. Polyphenols might inhibit endothelial NADPH oxidase (NOX), leading to a decrease in O2 − release and formation of peroxynitrite, thus preventing endothelial dysfunction [31]. Moreover, these compounds may activate endothelial nitric oxide synthase (eNOS), resulting in increase bioavailability of nitrogen monoxide (NO), the most potent endogenous vasodilator [32]. Therefore, we may speculate that the protective effects of G. verum extract on the maintenance of coronary vasodilator tone and coronary circulation may be attributed to the presence of total phenols.
Furthermore, we evaluated whether 4-week treatment with G. verum affects systemic redox homeostasis and alters systemic production of prooxidants, as well as the capacity of the antioxidant defense system. Our results showed that G. verum extract consumption exerted benefits referring to systemic redox state, leading to a drop in the plasma levels of O2 −, NO2 −, and TBARS. Moreover, we observed an increase in the activity of SOD, as the first line of cellular defense against oxidative injury, which is in line with a decrease in O2 − level. In fact, we may hypothesize that probably O2 − formed in mitochondria was converted to H2O2 in a reaction catalyzed by SOD. Our observation that there was no difference in the level of plasma H2O2 between groups is in accordance with unchanged CAT activity since the rise in H2O2 level would have led to a higher activity of CAT [33]. It has been proposed that polyphenols may preserve mitochondrial function and modulate redox signalling via inhibition of enzymes involved in ROS production such as xanthine oxidase (XO) and nicotinamide adenine dinucleotide phosphate-oxidase (NOX). Another way to exert antioxidant effect is via chelation of iron ions which catalyze several free radical-generating reactions [7].
In our research, I/R led to a rise in the generation of all cardiac prooxidants, which is consistent with the previous reports that during reperfusion of ischemic tissue the reversal of oxygen to oxygen-starved myocardium is associated with a burst of ROS generation. The main sources of prooxidants involve mitochondrial respiratory electron transport chain and activation of XO, resulting in enhanced production of very reactive species such as O2 − and H2O2 [34]. Nevertheless, pretreatment with G. verum extract was capable of preventing an increase in the levels of most of the markers of myocardial oxidative damage. Markedly, higher levels of TBARS, O2 −, and H2O2 in coronary venous effluent in the control group compared to the G. verum group were noticed after 30 min restoration of blood flow, thus suggesting that G. verum extract attenuated oxidative stress derived from the endocardium of the left ventricle and endothelium of the coronary circulation. Increased activity of myocardial antioxidant enzymes supports a decline in cardiac production of prooxidants. In fact, enhanced SOD activity might be responsible for a decrease in O2 − levels, while lower level of H2O2 may be explained by its decomposition to water and oxygen catalyzed by CAT. In our study, G. verum treatment was associated with enhanced both systemic and myocardial SOD activity and diminished systemic and cardiac production of O2 −, thus indicating that initial modulation of this antioxidant enzyme is involved in the action of G. verum. Consistent with these results, striking evidence indicate that polyphenols are able to activate endogenous antioxidant defense system, particularly SOD and CAT, thus alleviating oxidative stress-induced myocardial damage. In that sense, we may assume that this is one of the mechanisms through which polyphenol-containing extracts such as Galium verum extract may exert its antioxidant effects. Additionally, antioxidant potential of these natural compounds involves their ability to act as direct free radical scavengers as well [35, 36]. In addition to the aforementioned mechanisms, it has been known that flavonoids might alleviate I/R-induced oxidative stress by inhibiting the xanthine dehydrogenase/xanthine oxidase system [37]. Promising potential of G. verum extract in a decrease of oxidative damage may be mostly attributed to the additive and synergistic effects of bioactive molecules belonging to polyphenols.
Histological assessment suggested that ischemia altered the myocardial structure in both groups manifested as hypertrophia of cardiac muscle fibers, edema, necrosis, and degenerative changes. However, G. verum consumption alleviated I/R-induced deleterious effects on heart morphology and confirmed its potential to preserve both myocardial function and structure under ischemic conditions.
Our research highlighted for the first time that methanol extract of G. verum improved in vivo cardiac function and exerted benefits on systemic redox homeostasis. Additionally, it preserved the functional and morphological properties of the heart and prevented coronary vascular dysfunction after ischemia. Moreover, G. verum modulated the cardiac generation of prooxidants, thus alleviating oxidative stress-induced heart damage. Obvious protective effects of this plant species on myocardial I/R injury in our study provide a scientific basis for its use in triggering cardioprotection. However, further studies are certainly necessary for better understanding the underlying mechanisms.
Acknowledgments
The authors would like to express gratitude to the Faculty of Medical Sciences, University of Kragujevac for Grant No. JP 06/17.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The results of this manuscript were presented at the 8th International Congress of Pathophysiology – Satellite Symposium, held on September 03, 2018 in Kragujevac, Serbia.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Neri M., Riezzo I., Pascale N., Pomara C., Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediators of Inflammation. 2017;2017:14. doi: 10.1155/2017/7018393.7018393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausenloy D. J., Yellon D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. The Journal of Clinical Investigation. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalogeris T., Baines C. P., Krenz M., Korthuis R. J. Ischemia/reperfusion. Comprehensive Physiology. 2016;7(1):113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W., Wu N., Shu W., Jia D., Jia P. Pharmacological preconditioning and postconditioning with nicorandil attenuates ischemia/reperfusion-induced myocardial necrosis and apoptosis in hypercholesterolemic rats. Experimental and Therapeutic Medicine. 2015;10(6):2197–2205. doi: 10.3892/etm.2015.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffari M. S., Liu J. Y., Abebe W., Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. American Journal of Cardiovascular Disease. 2013;3(4):180–196. [PMC free article] [PubMed] [Google Scholar]
- 6.Testai L., Martelli A., Cristofaro M., Breschi M. C., Calderone V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. Journal of Pharmacy and Pharmacology. 2013;65(5):750–756. doi: 10.1111/jphp.12032. [DOI] [PubMed] [Google Scholar]
- 7.Debnath J., Nath L. K. A review on pathophysiology of ischemic-reperfusion injury of heart and ameliorating role of flavonoids and polyphenols. Journal of Medicinal Plants Research. 2014;8(16):607–614. doi: 10.5897/JMPR2014.5360. [DOI] [Google Scholar]
- 8.Lakić N. S., Mimica-Dukić N. M., Isak J. M., Božin B. N. Antioxidant properties of Galium verum L. (Rubiaceae) extracts. Central European Journal of Biology. 2010;5(3):331–337. doi: 10.2478/s11535-010-0022-4. [DOI] [Google Scholar]
- 9.Demirezer L. Ö., Gürbüz F., Güvenalp Z., Ströch K., Zeeck A. Iridoids, flavonoids and monoterpene glycosides from Galium verum subsp. verum . Turkish Journal of Chemistry. 2006;30:525–534. [Google Scholar]
- 10.Hijazi A., Al Masri D. S., Farhan H., Nasser M., Rammal H., Annan H. Effect of different ethanol concentrations, using different extraction techniques, on the antioxidant capacity of Lebanese Eryngium creticum . Journal of Pharmaceutical, Chemical and Biological Sciences. 2015;3:262–271. [Google Scholar]
- 11.Joffe I. I., Travers K. E., Perreault-Micale C. L., et al. Abnormal cardiac function in the streptozotocin-induced non–insulin-dependent diabetic rat: noninvasive assessment with Doppler echocardiography and contribution of the nitric oxide pathway. Journal of the American College of Cardiology. 1999;34(7):2111–2119. doi: 10.1016/S0735-1097(99)00436-2. [DOI] [PubMed] [Google Scholar]
- 12.Auclair C., Voisin E. Nitroblue tetrazolium reduction. In: Greenwald R. A., editor. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL, USA: CRC Press; 1985. pp. 123–132. [Google Scholar]
- 13.Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. Journal of Immunological Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 14.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. Journal of Biological Chemistry. 1969;244(22):6056–6063. [PubMed] [Google Scholar]
- 17.Beutler E. Catalase. In: Beutler E., editor. Red Cell Metabolism, a Manual of Biochemical Methods. New York, NY, USA: Grune and Stratton; 1982. pp. 105–106. [Google Scholar]
- 18.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105(25):121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 19.Misra H. P., Fridovich I. The role of superoxide-anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 20.Beutler E. Superoxide dismutase. In: Beutler E., editor. Red Cell Metabolism, a Manual of Biochemical Methods. Philadelphia, PA, USA: Grune and Stratton; 1984. pp. 83–85. [Google Scholar]
- 21.Beutler E. Reduced glutathione (GSH) In: Beutler E., editor. Red Cell Metabolism, a Manual of Biochemical Methods. New York, NY, USA: Grune and Stratton; 1975. pp. 112–114. [Google Scholar]
- 22.Zhang Y., Yuan C., Fang H., Li J., Su S., Chen W. Total flavonoid extract from Coreopsis tinctoria Nutt. protects rats against myocardial ischemia/reperfusion injury. Iranian Journal of Basic Medical Sciences. 2016;19(9):1016–1023. [PMC free article] [PubMed] [Google Scholar]
- 23.Mølgaard S., Faricelli B., Salomonsson M., Engstrøm T., Treiman M. Increased myocardial vulnerability to ischemia-reperfusion injury in the presence of left ventricular hypertrophy. Journal of Hypertension. 2016;34(3):513–523. doi: 10.1097/HJH.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 24.Pagliaro P., Penna C. Hypertension, hypertrophy, and reperfusion injury. Journal of Cardiovascular Medicine. 2017;18(3):131–135. doi: 10.2459/JCM.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 25.Vlase L., Mocan A., Hanganu D., Benedec D., Gheldiu A., Crișan G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae) Digest Journal of Nanomaterials and Biostructures. 2014;9(3):1085–1094. [Google Scholar]
- 26.Randriamboavonjy J. I., Loirand G., Vaillant N., et al. Cardiac protective effects of Moringa oleifera seeds in spontaneous hypertensive rats. American Journal of Hypertension. 2016;29(7):873–881. doi: 10.1093/ajh/hpw001. [DOI] [PubMed] [Google Scholar]
- 27.Yan L., Zhang J. D., Wang B., et al. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity. PLoS One. 2013;8(9, article e72548) doi: 10.1371/journal.pone.0072548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung L., Martin J. B., Lawmaster T., Arthur K., Broderick T. L., Al-Nakkash L. Sex-dependent effects of dietary genistein on echocardiographic profile and cardiac GLUT4 signaling in mice. Evidence-Based Complementary and Alternative Medicine. 2016;2016:10. doi: 10.1155/2016/1796357.1796357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanno Y., Watanabe R., Zempo H., Ogawa M., Suzuki J., Isobe M. Chlorogenic acid attenuates ventricular remodeling after myocardial infarction in mice. International Heart Journal. 2013;54(3):176–180. doi: 10.1536/ihj.54.176. [DOI] [PubMed] [Google Scholar]
- 30.Srimachai S., Devaux S., Demougeot C., et al. Bacopa monnieri extract increases rat coronary flow and protects against myocardial ischemia/reperfusion injury. BMC Complementary and Alternative Medicine. 2017;17(1):p. 117. doi: 10.1186/s12906-017-1637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhlaghi M., Bandy B. Mechanisms of flavonoid protection against myocardial ischemia–reperfusion injury. Journal of Molecular and Cellular Cardiology. 2009;46(3):309–317. doi: 10.1016/j.yjmcc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Duarte J., Francisco V., Perez-Vizcaino F. Modulation of nitric oxide by flavonoids. Food & Function. 2014;5(8):1653–1668. doi: 10.1039/C4FO00144C. [DOI] [PubMed] [Google Scholar]
- 33.Petkovic A. M., Jakovljevic V. L., Bradic J. V., et al. The effects of potassium cyanide on the functional recovery of isolated rat hearts after ischemia and reperfusion: the role of oxidative stress. Oxidative Medicine and Cellular Longevity. 2018;2018:10. doi: 10.1155/2018/5979721.5979721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granger D. N., Kvietys P. R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biology. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattera R., Benvenuto M., Giganti M., et al. Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients. 2017;9(5):p. 523. doi: 10.3390/nu9050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis X. L., Thandapilly S. J., Kalt W., et al. Blueberry polyphenols prevent cardiomyocyte death by preventing calpain activation and oxidative stress. Food & Function. 2014;5(8):1785–1794. doi: 10.1039/C3FO60588D. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y. W., Chou H. C., Lin S. T., et al. Cardioprotective effects of quercetin in cardiomyocyte under ischemia/reperfusion injury. Evidence-Based Complementary and Alternative Medicine. 2013;2013:16. doi: 10.1155/2013/364519.364519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.