Highlights
-
•
Systemic GHD causes behavioral hypersensitivity at P7 and P14, but not P21.
-
•
Primary afferent sensitization is observed in GHRHr KOs.
-
•
Knockout of GHRHr changes DRG gene expression that is observed throughout development.
Keywords: Neonatal, Dorsal root ganglion, Pain, Electrophysiology, Molecular biology, Growth hormone
Abstract
Injury during early postnatal life causes acute alterations in afferent function and DRG gene expression, which in addition to producing short-term sensitivity has the potential to influence nociceptive responses in adulthood. We recently discovered that growth hormone (GH) is a key regulator of afferent sensitization and pain-related behaviors during developmental inflammation of the skin. Peripheral injury caused a significant reduction in cutaneous GH levels, which corresponded with the observed hypersensitivity. However, it has yet to be determined whether GH deficiency (GHD) is sufficient to drive peripheral sensitization in uninjured animals. Here, we found that systemic GHD, induced by knockout of the GH release hormone receptor (GHRHr), was able to induce behavioral and afferent hypersensitivity to peripheral stimuli specifically during early developmental stages. GHD also produced an upregulation of many receptors and channels linked to nociceptive processing in the DRGs at these early postnatal ages (P7 and P14). Surprisingly, P21 GHRHr knockouts also displayed significant alterations in DRG gene expression even though behavioral and afferent hypersensitivity resolved. These data support previous findings that GH is a key modulator of neonatal hypersensitivity. Results may provide insight into whether GH treatment may be a therapeutic strategy for pediatric pain.
Introduction
Growth hormone (GH) is a signaling molecule whose primary action is to stimulate growth and regulate metabolic functions (Lin-Su and Wajnrajch, 2002). GH release is mainly initiated by signaling from growth hormone releasing hormone (GHRH), which is produced in the hypothalamus and acts on its receptor in the pituitary gland. After release, GH can directly influence target tissues via the GH receptor (GHr), which modulates many intracellular pathways and stimulates the production of insulin-like growth factor-1 (IGF-1). IGF-1 is a key mediator of GH actions on growth (Devesa et al., 2016).
There is some clinical evidence that GH may also be an important regulator of pain. Both growth and post-injury tissue repair are influenced by the GH signaling cascade (Lanning and Carter-Su, 2007; Rosenfeld and Hwa, 2009). In addition, there have been a few recent reports that patients with growth hormone deficiency (GHD) often have a resting pain in their limbs (Cimaz et al., 2001; Bennett, 2004). Furthermore, subpopulations of patients with fibromyalgia (FM) display reduced levels of GH. Diminished FM pain is achieved in these patients when treating with exogenous GH (Cuatrecasas et al., 2012, Cuatrecasas et al., 2010). Other reports have also shown that children with pain from cutaneous ulcers or erythromelalgia (Cimaz et al., 2001; Dr. John Rose, Cincinnati Children’s Hospital, personal communication) that concurrently have GHD show pain relief after a GH therapy.
Recent preclinical evidence has shown that upstream regulators of GH release can blunt hypersensitivity after peripheral injuries (Garcia et al., 2008; Talhouk et al., 2004; Sibilia et al., 2006). We have further found that cutaneous levels of GH appear to be transiently reduced after peripheral inflammation, which corresponded to observed hypersensitivity and subsequent recovery in neonatal mice (Liu et al., 2017). Pre-treatment of injured mice with low dose, exogenous GH was able to reverse the observed behavioral hypersensitivity and sensitization of primary afferents. This low dose and transient GH treatment, however, did not alter the levels of many inflammatory mediators in the periphery or induce any effects on growth. One possible mechanism by which peripheral GH appeared to take effect was through dynamic regulation of DRG insulin growth factor-1 receptor (IGFr1) expression.
Early neonatal life is a period filled with a multitude of normal developmental changes in the sensory system. Neurochemical and functional modifications take place during this time (Fitzgerald and Beggs, 2001; Luo et al., 2007, 2009; Molliver et al., 1997; Jankowski et al., 2014; Ye and Woodbury, 2010). Injury during early postnatal development has the potential to cause acute alterations in sensory function and DRG gene expression (Jankowski et al., 2014; Boada et al., 2011, 2010; Ririe et al., 2008; Vega-Avelaira et al., 2009; Nandi et al., 2004). These changes have the potential to restructure the developing central nociceptive system, and lead to hypersensitivity, which can persist into adulthood upon re-injury (Moriarty et al., 2018; Beggs et al., 2012; Cignacco et al., 2009; Fitzgerald and Walker, 2009; Walker et al., 2009; Ren et al., 2004). Considering the implications of peripheral injury during neonatal periods and the effects of GH on neonatal inflammatory pain (Liu et al., 2017), it is important to determine whether a reduction in GH alone is sufficient to drive peripheral hypersensitivity and modulate functional sensory development. In our current study, we used the growth hormone releasing hormone receptor knockouts (GHRHr KO) as a preclinical model of GHD (Beamer and Eicher, 1976; Eicher and Beamer, 1976; Jansson et al., 1986; Gaylinn et al., 1999). We tested the hypothesis that a GH deficient state is sufficient to induce behavioral hypersensitivity as well as primary afferent sensitization during early postnatal development.
Experimental Procedures
Animals
A total of 206 male and female wild type (WT) C57BL/6, and homozygous (−/−) or heterozygous (+/−) growth hormone releasing hormone receptor knockout (GHRHr KO) mice were used in all studies. Mice ranged in age from postnatal day 6-22. The GHRHr KO mice were obtained from Jax (C57BL/6J-Ghrhrlit/J; Stock#: 000533). Mice were kept with the mother and housed in a barrier facility on a 12-hour light/dark cycle at Cincinnati Children’s Hospital Medical Center (CCHMC), where they were given food and water ad libitum. All procedures were approved by the CCHMC Institutional Animal Care and Use Committee and were compliant with AALAC approved practices.
Behavioral analyses
All animals were habituated to the testing environment, which consisted of individual small boxes in a climate-controlled barrier facility, for at least 15 min prior to testing. Both heat and mechanical sensitivity were assayed in all groups at each defined age. Mechanical thresholds were first determined for P7-P14 mice by application of a series of Von Frey (VF) filaments of increasing force to the dorsal surface of the hindpaw in order to elicit a withdrawal response according to our previous procedures (Liu et al., 2017) and as originally described by Marsh et al (1999). Briefly, the medial dorsal surface of the hindpaw (which is innervated by the saphenous nerve) was probed with the VF filament with enough force to bend for 1-2 s. Thresholds to withdrawal were obtained over three rounds with 5-minintervals separating each round. The 4 g weighted VF filament was the maximum fiber used. Average withdrawal thresholds from the three rounds of testing were calculated for each mouse and then averaged among animals within a group prior to comparison. For animals at P21, mechanical thresholds were determined using a digital Randall-Selitto device (IITC Inc.) as hairy skin VF testing is not feasible in these older animals. Steady increasing pressure was applied to the medial dorsal surface of the hindpaw skin until a paw withdrawal was elicited. Cutoff intensity was set at a maximum of 300 gm.
Following VF (P7 and P14) or Randall-Selitto (P21) testing, mice then underwent a water bath test for heat hypersensitivity as previously described (Liu et al., 2017, Marsh et al., 1999, Walker et al., 2003). Both hind paws were submerged into the water and latency to withdrawal was measured, with a cut off time of 60 s. P7 mice were tested at 40 °C and 45 °C, while P14 and P21 mice were tested at 45 °C and 50 °C, due to different maximal temperatures being required at different developmental ages (Marsh et al., 1999, Walker et al., 2003). All testing was again performed over three rounds with 5-minintervals separating each round. The average of these trials was then calculated for each mouse and averaged across mice within a group. All values were reported as mean ± SEM. Direct comparisons of cohorts from littermate and non-littermate WT mice confirm that these distinct WTs were not different from each other. Regardless, experimenters were blinded to genotype in all instances.
Ex vivo preparation
We performed our ex vivo cutaneous afferent preparation as described previously (Liu et al., 2017, Jankowski et al., 2014, Jankowski et al., 2009a, Jankowski et al., 2009b). Briefly, mice were deeply anesthetized with an intramuscular hindlimb injection of 90 mg/kg ketamine and 10 mg/kg xylazine and perfused intracardially with chilled, oxygenated artificial cerebrospinal fluid (aCSF; in mM: 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-glucose) containing 253.9 mM sucrose instead of NaCl. The spinal cord (SC) and the right hindlimb were excised and placed in a circulating bath of the same solution. The SC was then hemisected and the saphenous nerve, along with the hairy hindpaw skin it innervates was dissected in continuity with the L1-5 dorsal root ganglia (DRGs). Following dissection, the tissue was transferred to a recording chamber containing circulating aCSF in which the sucrose was replaced with 127.0 mM NaCl. The skin was pinned out on an elevated platform, keeping the epidermis dry and the dermis perfused, and the chamber was gradually warmed to 32 °C.
Quartz microelectrodes (impedance >150 MΩ) containing 5% Neurobiotin (Vector Laboratories, Burlingame, CA) in 1 M potassium acetate were used in order to intracellularly record from L2 or L3 DRG somata. Once an electrically driven cell was found via suction electrode stimulation on the side of the saphenous nerve, the hindpaw skin was stimulated with a brush until the location of the receptive field (RF) was found. If a cell was driven by the nerve but had no mechanical RF, hot (˜53 °C) and/or cold (˜1 °C) physiological saline was applied to the skin to test thermal responsiveness.
The responses of individual DRG cells were then characterized by first testing for mechanical and then thermal responses. An increasing series of Von Frey hairs (0.07 g to 10 g) were used to test mechanical sensitivity by gently probing the RF of the cell for 1-2 s. Thermal stimuli were then applied to the RF of the hairy hindpaw skin using a 3 × 5 mm contact area peltier element (Yale Univ. Machine Shop) or physiological saline as described above. The peltier allowed for a controlled stimulus which consisted of a variable cold ramp that started at 31 °C and dropped to approximately 2-4 °C, which was maintained for about 3 s and slowly allowed to return to the bath temperature (32 °C). Bath temperature was held for a few seconds, followed by a heat ramp which produced an increasing heat stimulus to the RF up to 52 °C. The ramp rises in temperature from 32 °C to 52 °C over 12 s, at which point the 52 °C stimulus was held for 5 s and then the ramp returned the RF to 32 °C in 12 s. In between each application of mechanical and thermal stimuli, a recovery time of ˜20 seconds was given. No differences were found between those fibers recorded at the beginning from those obtained at the end of the experiment.
Afferent responses to the various stimuli were recorded and later, using Spike2 software (Cambridge Electronic Design), were characterized offline for conduction velocity, mean firing rate calculated within 200 ms bins, and mean peak instantaneous frequency to each stimulus type. Data are represented as mean ± SEM. A total of 320 cells were intracellularly recorded and physiologically characterized in the current study. The average number of cells recorded per condition/age was 53, which were obtained from an average of 6 mice per preparation. The minimum number of mice per preparation was 4 and a minimum of 46 cells were obtained from each of the 6 groups analyzed. The total numbers of animals and cells recorded per condition are listed in Table 1. No differences were found between WT and GHRHr +/− groups (see Table 2) and were thus combined into a single control group for ease of presentation and to increase statistical power for comparisons to GHRHr−/− mice. Similarly, since we were not able to obtain ample cell numbers from individual subtypes from each sex to make valid conclusions, these data are from both sexes.
Table 1.
Number of animals and total cell counts for electrophysiology experiments.
| Condition | ||
|---|---|---|
| P7 | Control | GHRHr−/− |
| #Mice | 5 | 7 |
| #Cells | 46 | 59 |
| P14 | Control | GHRHr−/− |
| #Mice | 6 | 8 |
| #Cells | 61 | 57 |
| P21 | Control | GHRHr−/− |
| #Mice | 4 | 5 |
| #Cells | 47 | 50 |
Table 2.
Electrophysiological data for WT and GHRHr+/− mice at P7, P14, and P21. No differences detected between groups. One-way ANOVA or one-way ANOVA on ranks.
| Mechanical Threshold (g) | Mechanical Firing Rate (Hz) | Mechanical Inst. Frequency (Hz) | Heat Threshold (oC) | Heat Firing Rate (Hz) | Mechanical Responders | Heat Responders | ||
|---|---|---|---|---|---|---|---|---|
| P7 | ||||||||
| All Cells | WT | 15.73 ± 4.70 | 6 ± 1.44 | 54.54 ± 11.48 | 35.75 ± 2.55 | 4 ± 0 | n = 11 | n = 2 |
| GHRHr+/− | 56 ± 10.99 | 3.94 ± 1.15 | 39.44 ± 14.22 | 47.96 ± 0 | 2 ± 0 | n = 18 | n = 1 | |
| HTMR | WT | 13.33 ± 6.01 | 4.33 ± 0.88 | 53.82 ± 12.29 | nd | nd | n = 3 | n = 0 |
| GHRHr+/− | 49 ± 21 | 3.5 ± 1.5 | 22.67 ± 13.16 | nd | nd | n = 4 | n = 0 | |
| CPM | WT | 13 ± 12 | 8 ± 6 | 53.42 ± 35.95 | 35.75 ± 2.55 | 4 ± 0 | n = 2 | n = 2 |
| GHRHr+/− | 3.67 ± 1.33 | 7 ± 0.58 | 45.99 ± 3.90 | 47.96 ± 0 | 2 ± 0 | n = 3 | n = 1 | |
| CM | WT | 25.33 ± 14.15 | 5.67 ± 4.18 | 41.75 ± 21.9 | nd | nd | n = 3 | n = 0 |
| GHRHr+/− | 70.13 ± 15.39 | 3.71 ± 2.39 | 62.95 ± 30.15 | nd | nd | n = 8 | n = 0 | |
| P14 | ||||||||
| All Cells | WT | 5 ± 0 | 5.6 ± 1.33 | 63.82 ± 10.20 | nd | nd | n = 5 | n = 0 |
| GHRHr+/− | 49.55 ± 8.03 | 5.07 ± 0.79 | 49.84 ± 7.48 | 41.16 ± 2.33 | 3.83 ± 1.51 | n = 28 | n = 6 | |
| HTMR | WT | 5 ± 0 (n = 1) | 4.67 ± 2.19 | 65.26 ± 14.22 | nd | nd | n = 3 | n = 0 |
| GHRHr+/− | 62.5 ± 37.5 | 3 ± 2 | 47.04 ± 46.29 | nd | nd | n = 2 | n = 0 | |
| CPM | WT | nd | nd | nd | nd | nd | n = 0 | n = 0 |
| GHRHr+/− | 40.83 ± 13.13 | 4.18 ± 0.71 | 41.38 ± 7.67 | 44.22 ± 0.95 | 4 ± 1.84 | n = 11 | n = 5 | |
| CM | WT | 5 ± 0 | 7 ± 0 | 41 ± 0 | nd | nd | n = 1 | n = 0 |
| GHRHr+/− | 81.25 ± 9.15 | 2.88 ± 0.69 | 30.87 ± 12.56 | nd | nd | n = 8 | n = 0 | |
| P21 | ||||||||
| All Cells | WT | 40.42 ± 8.44 | 6.08 ± 0.98 | 105.61 ± 25.48 | 41.97 ± 4.06 | 2.6 ± 1.12 | n = 27 | n = 5 |
| GHRHr+/− | 37.2 ± 12.39 | 4.9 ± 1.1 | 118.66 ± 52.08 | 47.24 ± 0.20 | 1.5 ± 0.5 | n = 10 | n = 2 | |
| HTMR | WT | 30.25 ± 23.35 | 6.75 ± 3.25 | 84.35 ± 29.97 | nd | nd | n = 4 | n = 0 |
| GHRHr+/− | 10 ± 0 | 3 ± 0 | 77.9 ± 0 | nd | nd | n = 1 | n = 0 | |
| CPM | WT | 40 ± 12.67 | 4.3 ± 0.87 | 37.5 ± 7.81 | 44.88 ± 3.65 | 2.75 ± 1.44 | n = 10 | n = 4 |
| GHRHr+/− | 36.67 ± 31.67 | 5.67 ± 2.33 | 42.68 ± 18.31 | 47.4 ± 0.20 | 1.5 ± 0.5 | n = 3 | n = 2 | |
| CM | WT | 69.17 ± 19.51 | 5.33 ± 1.89 | 55.73 ± 14.39 | nd | nd | n = 6 | n = 0 |
| GHRHr+/− | 66.67 ± 16.67 | 3 ± 1.15 | 23.71 ± 15.94 | nd | nd | n = 3 | n = 0 | |
RNA isolation, reverse transcription and realtime PCR
Animals were anesthetized with the ketamine/xylazine mixture as described above. The mice were then intracardially perfused with chilled (4 °C) 0.9% NaCl, followed by the dissection of DRGs. L2/ L3 DRG RNA was isolated using Qiagen RNeasy mini kits for animal tissues using the supplied protocol (n = 3-10 per condition and time point). A Nanodrop spectrometer (Thermo) was then used to determine RNA concentrations and purity. 1μg of total RNA was treated with DNase I (Invitrogen) and then DNased RNA was reverse transcribed using Superscript II Reverse Transcriptase (Invitrogen). For realtime PCR, 20 ng samples of cDNA were added to a SYBR Green Master Mix (Applied Biosystems) containing the appropriate primer combinations and run in duplicate on an Applied Biosystems Step-ONE realtime PCR machine. Primer sequences were obtained from Liu et al. (2017), Jankowski et al (2014), or Elitt et al (2006). Primer sequences for estrogen receptors (Esr) 1 and 2 are as follows: Esr1: Forward: 5’- ACTTGGAAGGCCGAAATG -3’; Reverse: 5’- GCAGGGCTATTCTTCTTAGTG -3’; Esr2: Forward: 5’- GCCATGATTCTCCTCAACTC -3’; Reverse: 5’- CTGTCACTGCGTTCAATAGG -3’. Cycle time (Ct) values were normalized to GAPDH and changes in expression are calculated as a ΔΔCt value that is determined by subtracting the Ct values of the gene of interest from the GAPDH internal control for each sample and compared among samples. Fold change is described as 2ΔΔCt (Applied Biosystems) and 2-fold change equals 100% change (mean ± SEM).
Immunocytochemistry
Mice were anesthetized as described with ketamine/xylazine and lumbar DRGs were isolated after cardiac perfusion with 0.9% NaCl. Individual DRGs were then immersion-fixed in 3% paraformaldehyde (in 0.1 M phosphate buffer (PB)) for 30 minutes, embedded in 10% gelatin (in PB) and post-fixed overnight in 3% paraformaldehyde. Following immersion in 20% sucrose, 45μm sections were obtained on a sliding microtome were then processed after blocking in 5% horse serum/ 5% goat serum/ 1% BSA/ 2.5% cold water fish skin gelatin/ 0.1% tween-20 (in PB) with primary antibodies against GH receptor (rabbit ant-GHr; AbCam, 1:1000) followed by CY3-conjugated secondary antibody binding (goat anti-rabbit-CY3; Jackson, 1:400). Sections were mounted on slides and cover slipped using Fluoromount-G containing 4′,6-diamidino-2-phenylindole (DAPI; Electron Microscopy Sciences, Hatfield, PA). Staining was visualized on a Nikon A1R GaAsP laser scanning confocal microscope at 40× or 100×, and images compiled using Adobe Photoshop.
Data analysis
Behavioral assays were compared using one-way analysis of variance (ANOVAs) with Holm Sidak post hoc tests after validating that data was determined to be normally distributed and of equal variance using Shapiro-Wilk and Brown-Forsythe tests, respectively. For instances where data was determined to not show normality or equal variance, non-parametric ANOVA on Ranks with Dunn’s post hoc test was used. We also performed Linear Mixed Effect models on conditions in which we obtained non-independent data (Aarts et al., 2014). These tests verify conclusions drawn from the other statistical analyses.
Peak firing rates (FR), mean peak instantaneous frequencies (IF), and thresholds to mechanical or heat stimuli were compared via one-way ANOVA with Holm-Sidak post hoc test or one-way ANOVA on Rank’s with Dunn’s post hoc and Mann-Whitney Rank Sum tests as appropriate. Percent change in gene expression changes in whole tissues were analyzed via one-way ANOVA with Tukey’s post hoc. In one instance, data was determined not to be normally distributed and this analysis was performed using Kruskal-Wallis and Dunn’s post hoc test. Critical significance level was defined at p<0.05. Rare instances of statistical outliers defined as values greater than two standard deviations away from the mean were not included in the analysis.
Results
Growth hormone release hormone receptor knockout mice (GHRHr KO) display age and sex dependent mechanical and heat hypersensitivity
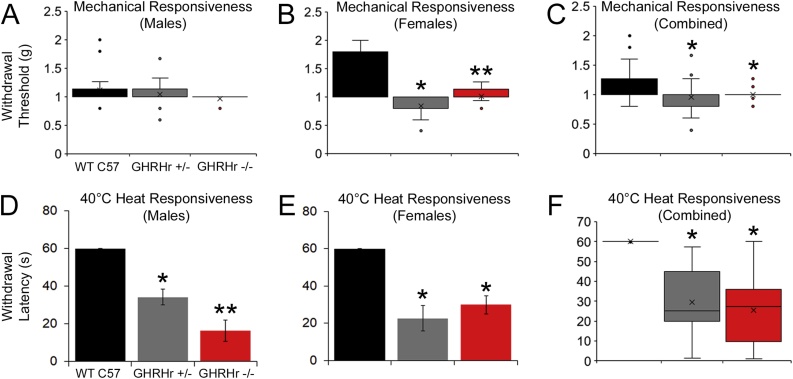
To determine if reduced GH levels were sufficient to drive hypersensitivity to peripheral stimuli, we first performed behavioral analyses in P7 GHRHr mutant mice (+/− and −/−) and compared them to WT C57Bl/6 controls. We then found in P7 male mice, that mechanical withdrawal thresholds were no different between WT C57 controls, GHRHr +/− knockouts or GHRHr −/− mutants (Fig. 1A), while P7 female GHRHr +/− and GHRHr −/− mutants did display decreased paw withdrawal thresholds versus WT (Fig. 1B). Overall, GHRHr +/− and GHRHr −/− both showed decreased thresholds to mechanical stimulation of the hairy skin at P7 compared to WT C57 controls (Fig. 1C). When measuring heat withdrawal latency to a 40 °C water bath, we found that male GHRHr +/− and GHRHr −/− displayed significantly decreased withdrawal latencies to heat compared to WT (Fig. 1D). Female GHRHr +/− and GHRHr −/− also displayed similar hypersensitivity to heat versus WT (Fig. 1E). Results were confirmed when combining both sexes (Fig. 1F).
Fig. 1.
Mechanical and heat responsiveness in GHRHr KO mice at P7.
A: Mechanical withdrawal thresholds to von frey filament stimulation of the hairy hindpaw skin in male mice was not different between WT C57, GHRHr+/− or GHRHr−/− (H18,22,12 3.6, p < 0.2) mice at P7. B: Female GHRHr+/− and GHRHr−/− mice however displayed reduced mechanical withdrawal thresholds compared to age-matched WT controls (H16,26, 26, 30.0, p < 0.001). C: Combining both sexes shows similar mechanical hypersensitivity in GHRHr+/− and GHRHr−/− mice compared to WT (H44, 38, 38 22.5, p < 0.01). D: Male GHRHr+/− (n = 11) and GHRHr−/− (n = 6) mice showed significantly reduced paw withdrawal latencies to heat stimuli at 40 °C relative to WT (n = 9; F2,23, 28.4, p < 0.001). E: Female mice showed similar heat hyper-responsiveness as males (GHRHr+/− (n = 8), GHRHr−/− (n = 13), WT (n = 13); F2,31, 21.6, p < 0.001). F: Overall, GHRHr knockout animals display heat hypersensitivity to WT controls (H22,19,19, 41.0, p < 0.001). Thermal responsiveness testing showed that the GHRHr KO mice were more hypersensitive than the heterozygotes, but only in males. * p < 0.01 vs WT; ** p < 0.05 vs WT and GHRHr+/−. One-way ANOVA with Holm-sidak post hoc test (parametric) or one-way ANOVA on Ranks’ with Dunn’s post hoc test (non-parametric) as appropriate.
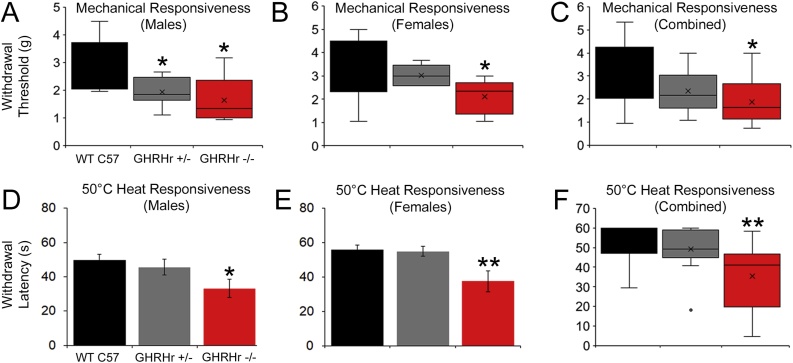
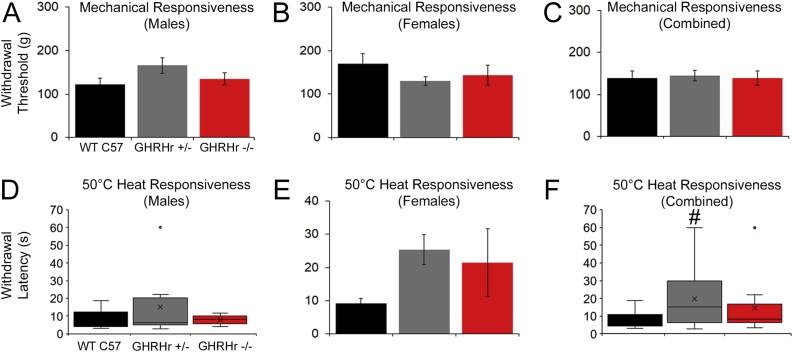
At P14, when assessing evoked responsiveness, GHRHr+/− and GHRHr−/− from both sexes displayed hypersensitivity to mechanical and heat stimuli compared to WTs. Mechanical thresholds of the male GHRHr+/− and GHRHr−/− were significantly lower than age-matched WT males. However, only GHRHr−/− females and not GHRHr+/− mice displayed significantly lower mechanical thresholds than the WT C57 s. Together, GHRHr−/− mice showed hypersensitivity to mechanical stimuli compared to WT (Fig. 2A-C). No sex differences were seen in response to a 50 °C water bath at P14; however only GHRHr−/− and not GHRHr+/− mice showed heat hypersensitivity compared to WT controls (Fig. 2D-F). At P21, however, we did not find any differences to mechanical or heat stimulation in GHRHr+/− or GHRHr−/− mice compared to WTs at P21 (Fig. 3).
Fig. 2.
Mechanical and heat sensitivity in GHRHr KO mice at P14.
A: At P14, male GHRHr+/− and GHRHr−/− mice now display reduced mechanical withdrawal thresholds relative to WT mice (H16,16,18, 16.9, p < 0.001). B: Female GHRHr−/− but not GHRHr+/− mice are also hyper-responsive to von frey filament stimulation of the hairy hindpaw skin at P14 compared to WT C57 animals (H12,10,18, 6.4, p < 0.05). C: Combined analysis reveals hyper-responsiveness to mechanical stimuli in GHRHr−/− mice at P14 (H30,28,36, 16.6, p < 0.001). Similar to P7 mice, male (D) and female (E) GHRHr −/− (Male: n = 9, F2,22, 3.6, p < 0.05; Female: n = 9, F2,20, 5.4, p < 0.02) mice display heat hypersensitivity relative to controls (Male: n = 8; Female: n = 9), though GHRHr +/− (Male: n = 8; Female: n = 5) mice do not. F: Combined data from both sexes is provided for reference (H17,13,18, 14.3, p < 0.001). * p < 0.05 vs. WT C57, ** p < 0.05 vs. WT and GHRHr +/−, one-way ANOVA with Holm-Sidak post hoc test (parametric) or one-way ANOVA on Ranks’ with Dunn’s post hoc test (non-parametric) as appropriate.
Fig. 3.
Mechanical and heat sensitivity in GHRHr KO mice at P21.
A-C: Male and female GHRHr+/− (Male: n = 9; Female: n = 17) and GHRHr−/− (Male: n = 5; Female: n = 5) mice show no differences in mechanical withdrawal threshold compared to WT controls (Male: n = 9; Female: n = 5) at P21 (Male: F2,47, 1.2, p = 0.33; Female: F2,24, 0.7, p = 0.5; Combined: F2,47, 0.05, p = 0.96). D-E: Similar results are also found regarding heat withdrawal latencies in which male and female GHRHr mutants are no different than WT C57 mice (Male: H9,9,5, 0.4, p = 0.83; Female: F2,24, 1.6, p < 0.3). F: GHRHr+/− were found to be different than WT overall but this was not found to be different than GHRHr−/− mice (Combined: H14,29,12, 6.6). # p < 0.04 vs WT but not GHRHr−/−. One-way ANOVA with Holm-Sidak post hoc test (parametric) or one-way ANOVA on Ranks’ with Dunn’s post hoc test (non-parametric) as appropriate.
The average weights of mice at P7 were no different between WT C57 controls (3.7 g ± 0.3 g), GHRHr heterozygous mutants (GHRHr+/−: 4.3 g ± 0.9 g) or GHRHr homozygous mutants (GHRHr−/−: 3.4 g ± 0.5 g; F2,10, 0.63, p < 0.6). This was again observed at P14: WT (6.0 g ± 0.0 g), GHRHr +/− (5.8 g ± 0.7 g), and GHRHr −/− (5.0 g ± 0.3 g; F2,14, 1.6, p < 0.3). However, we did find differences in overall weight between WT controls (9.5 g ± 0.5 g), GHRHr +/− (6.0 g ± 0.0 g) and GHRHr −/− (6.2 g ± 0.5 g; p < 0.03) at P21. Sex differences were not observed (not shown).
Systemic reduction in GH levels alter primary afferent function during early postnatal development
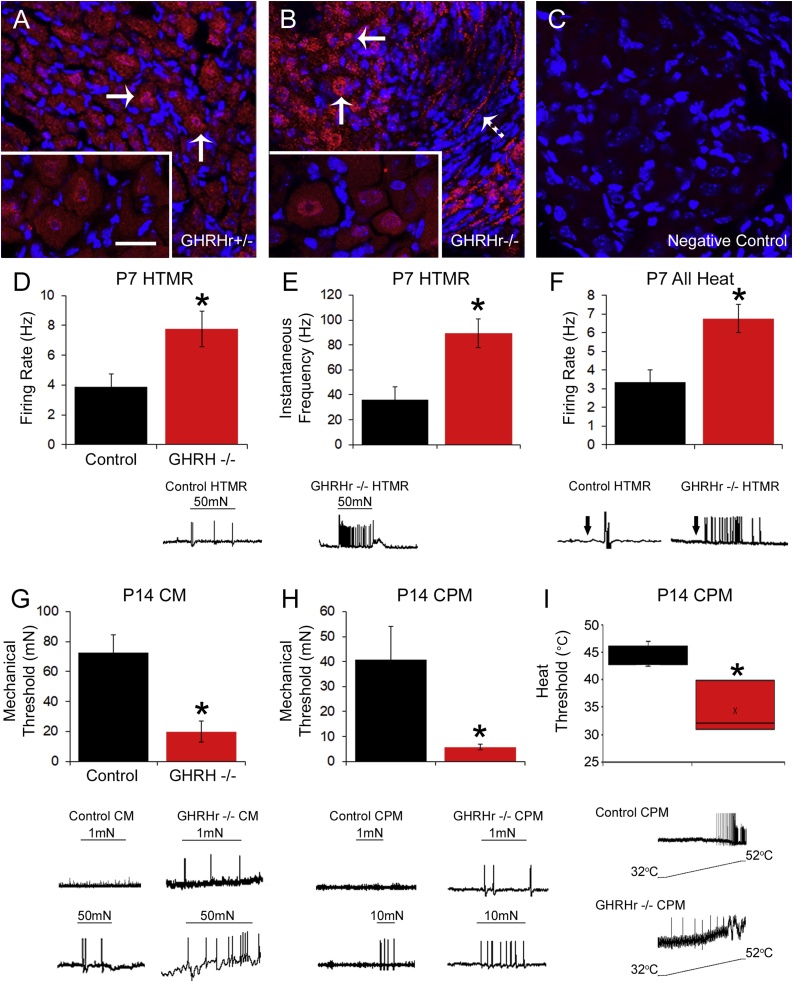
To begin to determine whether the behavioral effects of GHD were associated with alterations in the peripheral nervous system, we first stained the lumbar DRGs of GHRHr knockout mice for GH receptor (GHr). Immunocytochemical labeling indicates that GHr is present in many cells of the DRG including neurons (Fig. 4A-C). We then performed single unit recordings with our neonatal ex vivo hairy hindpaw skin/saphenous nerve/DRG/spinal cord recording preparation. In these recordings, we were not able to assess sex differences of individual subtypes due to low numbers of cells obtained from each sex in each group, therefore individual subtype data is combined from both sexes. Nevertheless, consistent with behavioral results from combined sexes, primary afferents in P7 GHRHr−/− mice displayed significant mechanical and heat hyper-responsiveness compared to controls. When assessing all cells at P7, we found no differences between control mice and GHRHr−/− animals regarding mechanical responsiveness (Table 3). However, when analyzing individual subtypes, the myelinated, high threshold mechano-receptors (HTMRs), in GHRHr−/− mice showed a significant increase in their mechanical firing rates (Fig. 4D) and mean peak instantaneous frequencies (Fig. 4E) versus controls. We were not able to determine the specific sub-population that resulted in afferent heat hypersensitivity due to low total numbers of heat responders obtained among the various subtypes. Regardless, we did find overall that primary sensory neurons in GHRHr −/− mice displayed significantly enhanced firing to heat stimulation of the skin compared to controls (Fig. 4F; Table 3). GHRHr−/− afferents that responded to heat (89.3 ± 11.5 Hz) also displayed significantly increased mean peak instantaneous frequencies to heat stimulation versus controls (36.0 ± 10.5 Hz; F1,6, 6.2, p < 0.05). No differences in polymodal C-fibers (CPM) or mechanically sensitive, thermally insensitive C-fibers (CM) were found at P7 (Table 3).
Fig. 4.
GH receptor staining in DRGs and response properties of primary afferents in GHRHr KO mice as assessed with ex vivo recording at P7 and P14.
Immunocytochemical staining for GH receptor (GHr; red) in the lumbar DRGs of GHRHr+/− (A) and GHRHr−/− (B) mice show ample labeling in various cells including putative neurons (arrows). Some staining is also evident in axon bundles within the DRGs (dashed arrow in B). Higher magnification (100x) images (inserts in A and B) indicate some cells also contain nuclear GHr labeling in addition to cytoplasmic staining. Scale Bar in 100x insert, 20μm. Negative control staining (no primary antibody) indicates binding specificity of the antibody (C). DAPI staining was also used to mark nuclei (blue). Ex vivo electrophysiological analysis of P7 GHRHr−/− (n = 12) mice showed an increase in firing rate (F1,17, 5.2, p < 0.04) (D) and mean peak instantaneous frequency (IF; E) (F1,17, 15.4, p < 0.001) of myelinated HTMR mechanical responses relative to controls (n = 7). F: Combined analysis of all heat responsive primary afferents revealed significant increases in firing to heat stimulation of the skin in GHRHr−/− mice (n = 4) compared to control afferents (n = 3; F1,5, 10.6, p < 0.03). At P14, CM neurons were found to display significant reductions in mechanical thresholds in GHRHr−/− mice (n = 10) compared to controls (n = 9; F1,17, 15.9, p < 0.001), (G) while CPM neurons showed lower mechanical (H) [U10,8, 19.0, p = 0.05] and heat (I) [Control: n = 3; GHRHr−/−: n = 4; F1,5, 8.0, p < 0.04] thresholds in the GHRHr−/− animals vs controls. Example responses for each parameter are provide below each panel. * p < 0.05 vs. control, one-way ANOVA with Holm-Sidak post hoc test (parametric) or Mann-Whitney Rank Sum test (non-parametric) as appropriate.
Table 3.
Response properties from various cell types using ex vivo recording in control and GHRHr−/− mice. *p < 0.05 vs. control. One-way ANOVA with Tukey’s post hoc or one-way ANOVA on Rank’s with Dunn’s post hoc as appropriate.
| Mechanical Threshold (g) | Mechanical Firing Rate (Hz) | Mechanical Inst. Frequency (Hz) | Heat Threshold (oC) | Heat Firing Rate (Hz) | Mechanical Responders | Heat Responders | ||
|---|---|---|---|---|---|---|---|---|
| P7 | ||||||||
| All Cells | Controls | 40.72 ± 7.88 | 4.78 ± 0.90 | 47.76 ± 8.93 | 39.82 ± 4.33 | 3.33 ± 0.67 | n = 29 | n = 3 |
| GHRHr−/− | 24.06 ± 5.07 | 4.62 ± 0.56 | 69.07 ± 9.05 | 42.31 ± 2.24 | 6.75 ± 0.75* | n = 34 | n = 4 | |
| CPM | Controls | 7.40 ± 4.49 | 7.40 ± 1.94 | 48.97 ± 11.72 | 39.82 ± 4.33 | 3.33 ± 0.67 | n = 5 | n = 3 |
| GHRHr−/− | 15.50 ± 11.69 | 7.75 ± 1.25 | 62.49 ± 13.08 | 45.13 ± 2.17 | 7.5 ± 1.5 | n = 4 | n = 2 | |
| CM | Controls | 57.91 ± 13.09 | 4.40 ± 1.96 | 58.17 ± 18.71 | n = 11 | |||
| GHRHr−/− | 49.60 ± 12.35 | 2.40 ± 0.4 | 31.03 ± 10.17 | n = 10 | ||||
| P14 | ||||||||
| All Cells | Controls | 45.38 ± 7.63 | 5.15 ± 0.69 | 51.96 ± 6.55 | 41.16 ± 2.33 | 3.83 ± 1.51 | n = 33 | n = 6 |
| GHRHr−/− | 12.47 ± 2.88* | 6.21 ± 0.80 | 100.31 ± 13.43* | 39.76 ± 3.74 | 1.5 ± 0.29 | n = 34 | n = 5 | |
| HTMR | Controls | 43.33 ± 28.92 | 4.0 ± 1.41 | 57.97 ± 17.17 | nd | nd | n = 5 | n = 0 |
| GHRHr−/− | 12.83 ± 7.61 | 6.67 ± 2.54 | 107.03 ± 36.62 | nd | nd | n = 6 | n = 0 | |
| P21 | ||||||||
| All Cells | Controls | 39.53 ± 6.91 | 5.57 ± 0.73 | 107.42 ± 22.18 | 43.47 ± 2.97 | 2.29 ± 0.81 | n = 37 | n = 7 |
| GHRHr−/− | 37.50 ± 7.12 | 4.67 ± 0.59 | 107.82 ± 20.05 | 44.64 ± 1.79 | 2.27 ± 0.49 | n = 36 | n = 11 | |
When examining the primary afferents at P14, we found that cells in GHRHr −/− mice were hypersensitive overall to mechanical stimuli compared to controls (Table 3). Assessments of individual subpopulations indicated no differences in responsiveness to mechanical or heat stimuli among the myelinated HTMRs at P14 (Table 3), but we did observe significant alterations in the C-fiber populations. CM fibers displayed significant reductions in mechanical threshold in GHRHr−/− mice compared to controls (Fig. 4G). However, no statistical differences between control and GHRHr−/− CMs were found for either mean peak instantaneous frequencies (Control: 59.1 ± 7.7 Hz; GHRHr−/−: 32.0 ± 11.1 Hz; F1,17, 4.2, p < 0.06) or firing rates (Control: 3.3 ± 0.8 Hz; GHRHr−/−: 4.8 ± 1.2 Hz F1,17, 1.1, p < 0.32) to mechanical stimuli. Similarly, polymodal c-fibers (CPM) displayed significant reductions in mechanical threshold in GHRHr−/− mice compared to controls (Fig. 4H) but did not show any alterations in firing rate (Control: 4.1 ± 0.8 Hz; GHRHr−/−: 5.1 ± 0.7 Hz; F1,16, 0.9, p < 0.4) to mechanical stimuli. They did however display increased mean peak instantaneous frequencies to mechanical deformation of the skin (Control: 36.7 ± 6.7 Hz; GHRHr−/−: 64.9 ± 7.7 Hz; F1,16, 7.7, p < 0.02). GHRHr−/− CPM neurons also showed significantly reduced heat thresholds compared to controls (Fig. 4I). However, no differences in firing (FR: Control: 4.0 ± 1.8 Hz; GHRHr−/−: 1.4 ± 0.2 Hz; F1,8, 2.0, p < 0.2; IF: Control: 29.3 ± 13.6 Hz; GHRHr−/−: 8.3 ± 5.2 Hz; F1,8, 2.1, p < 0.2) to heat stimuli were observed between control (n = 5) and GHRHr−/− (n = 5) CPMs.
Consistent with behavioral results at P21, we found no differences in any primary afferent subtype between control and GHRHr−/− animals. Examples of select data from P21 primary afferent HTMRs and CPMs are provided for reference (Fig. 5; Table 3). We also did not observe any differences among all other fiber subtypes at any age between control and GHRHr −/− mice including the low threshold mechanoreceptors (D-Hairs or SA1) or mechanically sensitive, and cold sensitive C-fibers (CMC; not shown).
Fig. 5.
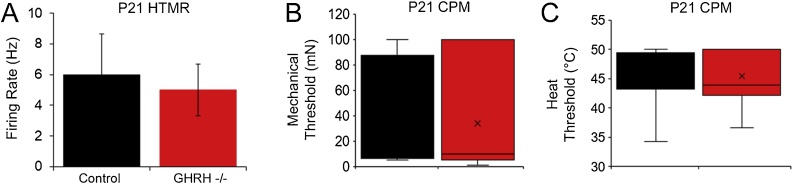
Response properties of primary afferents in GHRHr KO mice as assessed with ex vivo recording at P21.
Ex vivo analysis showed no differences in GHRHr−/− mice vs. controls in regard to firing to mechanical stimuli in HTMR neurons (A) [n = 5 per group; F1,8, 0.02, p < 0.91] or thresholds to mechanical (B) [H12,11, 0.4, p < 0.53] or heat (C) [H6,9, 0.0, p = 1.0] stimuli in CPM fibers. One-way ANOVA (parametric) or one-way ANOVA on Ranks’ (non-parametric) as appropriate.
Analysis of L2/L3 DRG gene expression in mice with GHD shows age related alterations
As GHD-induced hypersensitivity appeared to take effect at least in part through the primary afferents, we wanted to begin to determine some of the receptor mechanisms by which this could occur. We therefore performed realtime PCR on the L2/L3 DRGs from P7, P14 and P21 WTC57 and GHRHr−/− mice for a variety of sensory transducing receptors/channels (n = 3-10 per condition/age).
Interestingly at P7, of the tested genes, only insulin-like growth factor 1 receptor (IGFr1) and the estrogen receptors 1 (Esr1) and 2 (Esr2) were found to be different between GHRHr−/− and WTs. However, by P14, many genes were upregulated in the L2/L3 DRGs of GHRHr−/− mice. In addition to IGFr1, we found significant upregulation of acid sensing ion channel 3 (ASIC3), the ATP receptor, P2X3, mechanically sensitive channel, piezo2, heat channels, transient receptor potential (TRP) vanilloid type 1 (TRPV1) and TRP melastatin 3 (TRPM3), and ADP sensing G-protein coupled receptor, P2Y1. We further found significant upregulation of the artemin receptor, glial cell line-derived neurotrophic factor (GDNF) family receptor alpha 3 (GFRα3) and of the nerve growth factor receptor, trkA. We did not observe upregulation of the GDNF receptor, GFRα1, and Esr1 and Esr2 were no longer upregulated.
Surprisingly, at P21, many of the upregulated receptors remained elevated despite a restoration of behavior and afferent function to control levels at this age. Specifically, we found that P2X3, piezo2, TRPV1, P2Y1 and trkA remained increased in GHRHr−/− DRGs relative to WT. GFRα1 was also found to be upregulated in the mutants at this age (Table 4).
Table 4.
Percent change in gene expression in the L2/L3 DRGs of GHRHr−/− mice relative to WT at P7, P14 and P21. *p < 0.05, one-way ANOVA with Tukey’s post hoc test. ^data analyzed with Kruskal Wallis and Dunn’s post hoc tests.
| Gene | IGFr1 | ASIC3 | P2X3 | Piezo 2 | TRPV1 | TRPM3 | P2Y1 | GFRα1 | GFRα3 | trkA | Esr1 | Esr2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P7 | 208 ± 9%* | 21 ± 20% | 6 ± 31% | −5 ± 21% | 14 ± 19% | −4 ± 22% | −17 ± 17% | −16 ± 31% | 52 ± 22% | 50 ± 34% | 368 ± 9%* | 597 ± 26%* |
| P14 | 277 ± 12%* | 450 ± 51%* | 968 ± 21%* | 252 ± 16%* | 1529 ± 23%* | 323 ± 21%* | 41 ± 8%* | 5 ± 57% | 4673 ± 55%*,^ | 2686 ± 47%*,^ | −17 ± 22% | −51 ± 34% |
| P21 | 33 ± 28% | 9 ± 19% | 191 ± 10%* | 145 ± 11%* | 209 ± 10%* | 60 ± 23% | 168 ± 12%* | 155 ± 22%* | 57 ± 14% | 195 ± 6%* | −11 ± 21% | 65 ± 51% |
Discussion
Reports show that GHD can be associated with pain (Bennett, 2004; Cuatrecasas et al., 2010; Cimaz et al., 2001). We found that developing GHRHr −/− mice display mechanical and heat hypersensitivity in an age-related fashion (Fig. 1, Fig. 2, Fig. 3). In addition, systemic reduction in GH levels was able to alter primary afferent function that corresponded with results from behavioral experimentation (Fig. 4, Fig. 5). Results closely mimicked those obtained previously in mice with cutaneous injury (Jankowski et al., 2014; Liu et al., 2017), advancing evidence that GH is an important modulator of peripheral sensitivity during early life. Furthermore, gene expression analysis of the L2/L3 DRG in mice with GHD showed changes in expression of various sensory transducing receptors and modulators of neuronal development across the different developmental ages (Table 4). Surprisingly however, upregulation of certain genes was observed in the DRGs of older animals (P21), when both behavioral and afferent hypersensitivity returned to control levels. Hypersensitivity was also observed at ages (P7-P14) prior to when overt alterations in body weight are detected (P21) in mice with GHD. Results indicate that GH can regulate the functional development of peripheral sensitivity but that early life GH signaling may be able to influence sensory responsiveness later in life.
Much of our current knowledge of growth hormone action involves its role in growth and tissue repair (Devesa et al., 2017, 2016; Tuffaha et al., 2016). However, some reports have shown reduced GH levels in patients with cutaneous ulcers, erythromelalgia, and in subsets of FM patients corresponds with pain. Treatment of these patients with exogenous GH can relieve their pain (Cimaz et al., 2001; Cuatrecasas et al., 2012, 2010; Dr. John Rose, CCHMC, personal communication). Additional evidence suggests that GH may also be an adjunct pain therapy for patients with low back pain (Dubick et al., 2015). We recently reported that reduced GH levels may be important in driving hypersensitivity to peripheral inflammation in neonates (Liu et al., 2017). Here we examined whether a GHD state during development was alone sufficient to produce a pain-like state. We found that during both the first and second weeks of life, GHRHr −/− mice were behaviorally hypersensitive to evoked mechanical and thermal stimuli (Fig. 1,2). Interestingly, at P7, male mice showed no differences in mechanical hypersensitivity while females did (Fig. 1A, B). This difference was most likely the result of the GHRHr+/− females having a significantly lower withdrawal threshold than the heterozygous males, as the homozygous mutants did not differ between the sexes. Male GHRHr−/− did eventually display mechanical hypersensitivity by P14. Although to our knowledge, no sex specific effects of GHD are well-documented regarding pain, reports do indicate that GH may be a strong modulator of sensory function during early stages of life (Liu et al., 2017; Bennett 2004; Cimaz et al. 2001; Devesa et al., 2017). Results of our current report support this notion as by P21, GHRHr−/− mice did not display any evoked hypersensitivity like P7 and P14 animals (Fig. 1, Fig. 2, Fig. 3), though the necessary switch of tests for mechanical responsiveness at P21 does somewhat limit this interpretation. Our data also indicates an interesting time-dependent effect of GHD on sensory function that is differentially expressed in males and females. It is important to note that Esr1 and Esr2 were both upregulated specifically at P7 (Table 4). It will be interesting in the future to assess how GH signaling may influence or is responsive to alterations in sex hormone signaling.
Injury to neonates can also produce considerable functional changes in primary afferent responses (Jankowski et al., 2014; Boada et al., 2010, 2011; Ririe et al., 2008; Vega-Avelaira et al., 2009; Nandi et al., 2004). Specifically, injury during this period can cause age-specific sensitization of fast conducting, broad spiking “A”-fiber and slowly conducting, broad spiking “C”-fiber populations (Jankowski et al., 2014; Liu et al., 2017). Here we found that systemic GHD similarly caused age specific changes in these distinct subpopulations primary sensory neurons. As shown in previous studies (Jankowski et al., 2014; Liu et al., 2017), the A-fiber population became sensitized at P7, while at P14, C-fibers, specifically showed hypersensitivity to peripheral stimuli (Fig. 4; Table 3). Changes in afferent sensitivity at P7 were found specifically in regard to increased mechanical firing rate (FR) and mean peak instantaneous frequencies (IF) of the GHRHr−/− myelinated HTMR subpopulation. Increased heat FR was also observed, but this was only obtained when analyzing all fibers.
At P14, afferent sensitivity was not due to increased FR as previously reported after inflammation (Liu et al., 2017; Jankowski et al., 2014), but rather a decrease in the mechanical thresholds of CM and CPM fibers, as well as, a reduction in heat threshold of the CPMs. Distinct sensitization patterns may have arisen due to a strain difference as here, inbred C57Bl/6 lines were assessed, while in previous reports analyzing responses to inflammation, an outbred Swiss Webster line was used (Liu et al., 2017; Jankowski et al., 2014). It is also important to note that behaviorally, GHRHr+/− mice were different than WT controls (Fig. 1, Fig. 2). However, this appeared to be sex and modality specific. Due to low cell numbers obtained electrophysiologically from these different parameters, we are not able to fully confirm whether behavioral results are a result of specific alterations in the primary afferents. Confirmation of strain and sex-related effects on afferents in future experimentation will be important to fully appreciate a role for GH modulating sensory development. Nevertheless, we do observe a strong effect of GHD on sensory responsiveness specifically during early life.
Interestingly, even though we observed mechanical and thermal hyper-responsiveness both behaviorally and in the primary sensory neurons during the first two weeks of life, this subsided completely by P21 when assessing both of these aspects of peripheral sensitization (Fig. 3, Fig. 4, Fig. 5). This could imply that GH influences sensory function to a greater degree early in life, but potentially not by the time the sensory system is more functionally developed. This will again need to be explored further in future examination as we cannot fully rule out a role for the central nervous system in GHD-related hypersensitivity. Nonetheless, our data do support a role for the PNS in behavioral deficits observed in mice with GHD. Additionally, as these results may be similar to that observed after injury (Liu et al., 2017), it will be necessary in the future to explore the effect of peripheral injury in GHRHr KO mice, and whether early life hypersensitivity observed in these animals can modulate injury responses acutely or later in life (e.g. Moriarty et al., 2018).
Changes in gene expression in DRGs have been linked with altered sensory function after peripheral injury (Jankowski et al., 2010; Jankowski et al., 2012a; Jankowski et al., 2012b; Jankowski et al., 2014) and can help drive behavioral and afferent hypersensitivity (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5) (Jankowski et al., 2009a, 2009b, Jankowski et al., 2010 Wang et al., 2003; Wu et al., 2016). Behavioral and sensory neuron changes at the P7 time point, corresponded with an upregulation of IGFr1 in GHRHr−/− mice (Table 4). IGF-1 and IGFr1 have previously been established as potential modulators of hypersensitivity (Miura et al., 2011; Zhang et al., 2014). Even though systemic IGF-1 is at lower levels at some of the time points assessed (Donahue and Beamer, 1993), data may still suggest that IGFr1 is one player at this time point, and that dynamically altered levels of other targets, such as the TRP channels, or purinergic receptors are not related to GHD induced hypersensitivity. Of course, this does not indicate that IGFr1 is the only mechanism of GHD-related hypersensitivity in neonates. Although we did not assess other genes such as calcium channels, it is possible that these other factors are upregulated in GHD mice at this stage of development since IGFr1 has been shown to mediate its effects on afferent function through modulation of calcium channels (Zhang et al., 2014). Modulation of sodium or potassium channels may also be affected in our mutants, but this would need to be confirmed in the future. Interestingly, results at P7 somewhat differ from that observed in our previous reports in which mice experienced peripheral inflammation (Liu et al., 2017; Jankowski et al., 2014). Although IGFr1 was implicated in those and the current study (Table 4), other upregulated genes were not observed here at P7. The obvious difference between the current and the previous report is that mice were injured whereas here they were not. Many other mechanisms may be at play under conditions of peripheral inflammation (and GHD) and this should be investigated further to fully appreciate the interplay between GHD-related hypersensitivity and inflammation-induced nociceptive pain.
In contrast with P7 however, almost every gene examined at P14 was upregulated in the GHRHr−/− mice (Table 4). Two of the more pronounced changes in upregulation were TRPV1 and the artemin co-receptor, GFRα3. TRPV1 is a known heat channel (Caterina et al., 2000), while artemin has been associated with enhancing TRPV1 expression (Jankowski et al., 2010; Elitt et al., 2006). This corresponded well with behavioral results and ex vivo data (Fig. 2,4), as both measurements indicated heat hypersensitivity in GHRHr −/− mice. It is also possible that upregulation of P2Y1 could be responsible for changes in thermal sensitivity at the afferent level since we observed altered thresholds in the CPM fibers, and this receptor has been shown to regulate the thermal thresholds of the CPM population (Jankowski et al., 2012a). In addition, upregulation of genes associated with mechanical stimuli, such as ASIC3, P2 × 3, and piezo2 (Walder et al., 2010; Tsuda et al., 2000; Kim et al., 2012; Szczot et al., 2018) provide a good connection to the enhanced mechanical responsiveness seen behaviorally and in the primary sensory neurons. Direct manipulation of these individual genes will be necessary to fully confirm whether they drive the observed alterations in GHRHr −/− mice.
Surprisingly, many of the upregulated genes at P14 were also elevated at P21, even though behavior and afferent function were restored by this time. Results from gene expression analysis may thus challenge the notion that GH may more specifically influence functional sensory development during early life (Table 4). The gene changes we see at P14 that are also upregulated at P21 could be potential targets for influencing nociceptive processing in adulthood. Other than causing acute changes in sensory function, injury during the neonatal period also has been shown to have long-term implications. Disruption during an early life critical period can lead to a re-wiring of the nociceptive system in the spinal cord and upon re-injury cause hypersensitivity to persist for abnormally extended periods of time. This concept is one of the leading potential mechanisms behind the transition from acute to chronic neonatal pain (Moriarty et al., 2018; Cignacco et al., 2009; Fitzgerald and Walker, 2009; Walker et al., 2009; Ren et al., 2004; Beggs et al., 2012). Regarding GH signaling, the maintenance of P2X3 and Piezo2 in mice with GHD at P21 could shape mechanical hypersensitivity, while sustained upregulation of TRPV1 and P2Y1 may influence altered thermal responses in injured adults. Thus, it is conceivable that while IGFr1 could be one driver of acute hypersensitivity at P7 and P14, that GHD-related hypersensitivity in neonates could affect adult responses to peripheral injury through other upregulated genes such as P2X3, Piezo2, TRPV1, and P2Y1. This will be an important area of future investigation.
One curious result was the observed upregulation of the NGF receptor, trkA by P14. We and others have shown that trkA levels begin to decrease substantially after P0 and are also downregulated post neonatal injury (Jankowski et al., 2014; Luo et al., 2007, 2009; Molliver et al., 1997; Weskamp and Reichardt, 1991). Peripheral insults can delay the switch from NGF to GDNF responsiveness in sensory neurons of the DRG. Systemic GHD may also affect this phenotypic switch as it appears that it can postpone trkA downregulation and delay the upregulation of the GDNF co-receptor, GFRα1 (Table 4). Future analysis of the neurochemical development of the DRG will be therefore be warranted in GHRHr−/− animals.
Discovery of a safe but effective alternative analgesic for use in infants and children is particularly valuable. Our recent data suggest that GH may potentially fill this need. This is supported by multiple studies, which show that treatment of growth hormone deficient patients with GH can help improve pain outcomes (Cimaz et al., 2001; Bennett et al., 1992; Bagge et al., 1998; Jacobson et al., 1995; Bennett et al., 1998; Cuatrecasas et al., 2012). Considering the potentially harmful side effects of current analgesics, low dose growth hormone could be a useful option for the treatment of pediatric pain.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the NIH/NINDS (R56NS103179) and NIH/NICHD (R03HD077483) to MPJ. We would also like to thank Dr. John Rose for insight into this project and Dr. Lili Ding for statistical assistance.
References
- Aarts E., Verhage M., Veenvliet J.V., Dolan C.V., van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat. Neurosci. 2014;17:491–496. doi: 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Bagge E., Bengtsson B., Carlsson L., Carlsson J. Low growth hormone secretion in patients with fibromyalgia – a preliminary report on 10 patients and 10 controls. J. Rheumatol. 1998;25:145–148. [PubMed] [Google Scholar]
- Beamer W.H., Eicher E.M. Stimulation of growth in the little mouse. J. Endocrinol. 1976;71:37–45. doi: 10.1677/joe.0.0710037. [DOI] [PubMed] [Google Scholar]
- Beggs S., Currie G., Salter M.W., Fitzgerald M., Walker S.M. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. 2012;135:404–417. doi: 10.1093/brain/awr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R.M. Growth hormone in musculoskeletal pain states. Curr. Rheumatol. Rep. 2004;6:266–273. doi: 10.1007/s11926-004-0034-z. [DOI] [PubMed] [Google Scholar]
- Bennett R.M., Clark S.R., Burkhardt C.S., Walczyk J. A randomized, double-blind, placebo-controlled study of growth hormone in the treatment of fibromyalgia. Am. J. Med. 1998;104:227–231. doi: 10.1016/s0002-9343(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Bennett R.M., Clark S.R., Campbell S., Burkhardt C.S. Low levels of somatomedin c in patients with the fibromyalgia syndrome. Arthritis Rheum. 1992;35:1113–1116. doi: 10.1002/art.1780351002. [DOI] [PubMed] [Google Scholar]
- Boada M.D., Houle T.T., Eisenach J.C., Ririe D.G. Differing neurophysiologic mechanosensory input from glabrous and hairy skin in juvenile rats. J. Neurophysiol. 2010;104:3568–3575. doi: 10.1152/jn.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada M.D., Gutierrez S., Houle T., Eisenach J.C., Ririe D.G. Developmental differences in peripheral glabrous skin mechanosensory nerve receptive field and intracellular electrophysiologic properties: phenotypic characterization in infant and juvenile rats. Int. J. Dev. Neurosci. 2011;29:847–854. doi: 10.1016/j.ijdevneu.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cimaz R., Rusconi R., Fossali E., Careddu P. Unexpected healing of cutaneous ulcers in a short child. Lancet. 2001;358:211–212. doi: 10.1016/S0140-6736(01)05413-7. [DOI] [PubMed] [Google Scholar]
- Cignacco E., Hamers J., van Lingen R.A., Stoffel L., Büchi S., Müller R., Schütz N., Zimmermann L., Nelle M. Neonatal procedural pain exposure and pain management in ventilated preterm infants during the first 14 days of life. Swiss Med Weekly. 2009;139:226–232. doi: 10.4414/smw.2009.12545. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas G., Alegre C., Fernandez-Solà J., Gonzalez M.J., Garcia-Fructuoso F., Poca-Dias V., Nadal A., Cuatrecasas G. Growth hormone treatment for sustained pain reduction and improvement in quality of life in severe fibromyalgia. Pain. 2012;153:1382–1389. doi: 10.1016/j.pain.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas G., Gonzalez M.J., Alegre C., Sesmilo G., Fernandez-Solà J., Casanueva F.F., Garcia-Fructuoso F., Poca-Dias V. High prevalence of growth hormone deficiency in severe fibromyalgia syndromes. J. Clin. Endocrinol. Metab. 2010;95:4331–4337. doi: 10.1210/jc.2010-0061. [DOI] [PubMed] [Google Scholar]
- Devesa J., Almengló C., Devesa P. Multiple effects of growth hormone in the body: Is it really the hormone for growth? Clin. Med. Insights Endocrinol. Diabetes. 2016;9:47–71. doi: 10.4137/CMED.S38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devesa J., Alonso A., Lopez N., Garcia J., Puell C.I., Pablos T., Devesa P. Growth hormone (GH) and rehabilitation promoted distal innervation in a child affected by caudal regression syndrome. Int. J. Mol. Sci. 2017;18:230. doi: 10.3390/ijms18010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue L.R., Beamer W.G. Growth hormone deficiency in the ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1, or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- Dubick M.N., Ravin T.H., Michel Y., Morrisette D.C. Use of localized human growth hormone and testosterone injections in addition to manual therapy and exercise for lower back pain: a case series with 12-month follow up. J. Pain Res. 2015;8:295–302. doi: 10.2147/JPR.S81078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher E.M., Beamer W.G. Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J. Hered. 1976;67:87–91. doi: 10.1093/oxfordjournals.jhered.a108682. [DOI] [PubMed] [Google Scholar]
- Elitt C.M., McIlwrath S.L., Lawson J.J., Malin S.A., Molliver D.C., Cornuet P.K., Koerber H.R., Davis B.M., Albers K.M. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J. Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Walker S.M. Infant pain management: a developmental neurobiological approach. Nat. Clin. Pract. Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- Garcia J.M., Cata J.P., Dougherty P.M., Smith R.G. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–460. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylinn B.D., Dealmeida V.I., Lyons C.E., Jr, Wu K.C., Mayo K.E., Thorner M.O. The mutant growth hormone-releasing hormone (GHRH) receptor of the little mouse does not bind GHRH. Endocrinology. 1999;140:5066–5074. doi: 10.1210/endo.140.11.7092. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Main K., Danneskiold-Samsoe B., Skakkebaek N.E. A controlled study of serum insulin-like growth factor-one and urinary excretion of growth hormone in fibromyalgia. J. Rheumatol. 1995;22:1138–1140. [PubMed] [Google Scholar]
- Jankowski M.P., Lawson J.J., McIlwrath S.L., Rau K.K., Anderson C.E., Albers K.M., Koerber H.R. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J. Neurosci. 2009;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M.P., McIlwrath S.L., Jing X., Cornuet P.K., Salerno K.M., Koerber H.R., Albers K.M. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M.P., Rau K.K., Soneji D.J., Anderson C.E., Koerber H.R. Enhanced artemin/GFRalpha3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J. Neurosci. 2010;30:16272–16283. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M.P., Rau K.K., Soneji D.J., Ekmann K.M., Anderson C.E., Molliver D.C., Koerber H.R. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012;153:410–419. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M.P., Soneji D.J., Ekmann K.M., Anderson C.E., Koerber H.R. Dynamic changes in heat transducing channel TRPV1 expression regulate mechanically insensitive, heat sensitive C-fiber recruitment after axotomy and regeneration. J. Neurosci. 2012;32:17869–17873. doi: 10.1523/JNEUROSCI.3148-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M.P., Ross J.L., Weber J.D., Lee F.B., Shank A.T., Hudgins R.C. Age-dependent sensitization of cutaneous nociceptors during developmental inflammation. Mol. Pain. 2014;10:34. doi: 10.1186/1744-8069-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson J.O., Downs T.R., Beamer W.G., Frohman L.A. Receptor-associated resistance to growth hormone-releasing factor in dwarf "little" mice. Science. 1986;232:511–512. doi: 10.1126/science.3008329. [DOI] [PubMed] [Google Scholar]
- Lanning N.J., Carter-Su C. Recent advances in growth hormone signaling. Rev. Endocr. Metab. Disord. 2007;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- Lin-Su K., Wajnrajch M.P. Growth hormone releasing hormone and the GHRH receptor. Rev. Endocr. Metab. Disord. 2002;3:313–323. doi: 10.1023/a:1020949507265. [DOI] [PubMed] [Google Scholar]
- Liu X., Green K.J., Ford Z.K., Queme L.F., Lu P., Ross J.L., Lee F.C., Shank A.T., Hudgins R.C., Jankowski M.P. Growth hormone regulates the sensitization of developing peripheral nociceptors during cutaneous inflammation. Pain. 2017;158:333–345. doi: 10.1097/j.pain.0000000000000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Wickramasinghe S.R., Savitt J.M., Griffin J.W., Dawson T.M., Ginty D.D. A hierarchical NGF signaling cascade controls ret-dependent and ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Luo W., Enomoto H., Rice F.L., Milbrandt J., Ginty D.D. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.E., Coste B., Chadha A., Cook B., Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D., Dickenson A., Hatch D., Fitzgerald M. Epidural opioid analgesia in infant rats II: responses to carrageenan and capsaicin. Pain. 1999;82:33–38. doi: 10.1016/S0304-3959(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Miura M., Sasaki M., Mizukoshi K., Shibasaki M., Izumi Y., Shimosato G., Amaya F. Peripheral sensitization caused by insulin-like growth factor 1 contributes to pain hypersensitivity after tissue injury. Pain. 2011;152:888–895. doi: 10.1016/j.pain.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Molliver D., Wright D., Leitner M., Parsadanian A.S., Doster K., Wen D., Yan Q., Snider W. IB4-Binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Moriarty O., Harrington L., Beggs S., Walker S.M. Opioid analgesia and the somatosensory memory of neonatal surgical injury in the adult rat. Br. J. Anaesth. 2018;121:314–324. doi: 10.1016/j.bja.2017.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi R., Beacham D., Middleton J., Koltzenburg M., Howard R.F., Fitzgerald M. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain. 2004;111:38–50. doi: 10.1016/j.pain.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Ren K., Anseloni V., Zou S.P., Wade E.B., Novikova S.I., Ennis M., Traub R.J., Gold M.S., Dubner R., Lidow M.S. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ririe D.G., Liu B., Clayton B., Tong C., Eisenach J.C. Electrophysiologic characteristics of large neurons in dorsal root ganglia during development and after hind paw incision in the rat. Anesthesiology. 2008;109:111–117. doi: 10.1097/ALN.0b013e31817c1ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld R.G., Hwa V. The growth hormone cascade and its role in mammalian growth. Horm. Res. 2009;71:36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- Sibilia V., Lattuada N., Rapetti D., Pagani F., Vincenza D., Bulgarelli I., Locatelli V., Guidobono F., Netti C. Ghrelin inhibits inflammatory pain in rats: involvement of the opioid system. Neuropharmacology. 2006;51:497–505. doi: 10.1016/j.neuropharm.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Szczot M., Liljencrantz J., Ghitani N., Barik A., Lam R., Thompson J.H., Bharucha-Goebel D., Saade D., Necaise A., Donkervoort S., Foley A.R., Gordon T., Case L., Bushnell M.C., Bonnemann C.G., Chesler A.T. Piezo2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 2018;10:462. doi: 10.1126/scitranslmed.aat9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R.S., Saadé N.E., Mouneimne G., Masaad C.A., Safieh-Garabedian B. Growth hormone releasing hormone reverses endotoxin-induced localized inflammatory hyperalgesia without reducing the upregulated cytokines, nerve growth factor and gelatinase activity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2004;28:625–631. doi: 10.1016/j.pnpbp.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Koizumi S., Kita A., Shigemoto Y., Ueno S., Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J. Neurosci. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffaha S.H., Budihardjo J.D., Sarhane K.A., Khusheim M., Song D., Broyles J.M., Salvatori R., Means K.R., Jr., Higgins J.P., Shores J.T. Growth hormone therapy accelerates axonal regeneration, promotes motor reinnervation, and reduces muscle atrophy following peripheral nerve injury. Plast. Reconstr. Surg. 2016;137:1771–1780. doi: 10.1097/PRS.0000000000002188. [DOI] [PubMed] [Google Scholar]
- Vega-Avelaira D., Geranton S.M., Fitzgerald M. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Mol Pain. 2009;5:70. doi: 10.1186/1744-8069-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R.Y., Rasmussen L.A., Rainier J.D., Light A.R., Wemmie J.A., Sluka K.A. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J. Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.M., Meredith-Middleton J., Cooke-Yarborough C., Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Walker S.M., Tochiki K.K., Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain. 2009;147:99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Wang R., Guo W., Ossipov M.H., Vanderah T.W., Porreca F., Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Reichardt L.F. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Wu S., Marie L.B., Miao X., Liang L., Mo K., Chang Y.J., Du P., Soteropoulos P. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol. Pain. 2016;12:1–12. doi: 10.1177/1744806916629048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Woodbury C.J. Early postnatal loss of heat sensitivity among cutaneous myelinated nociceptors in Swiss-Webster mice. J. Neurophysiol. 2010;103:1385–1396. doi: 10.1152/jn.00472.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qin W., Qian Z., Liu X., Wang H., Gong S., Sun Y.-G., Snutch T.P., Jiang X., Tao J. Peripheral pain is enhanced by insulin-like growth factor 1 through a G protein-mediated stimulation of T-type calcium channels. Sci. Signal. 2014;7:1–14. doi: 10.1126/scisignal.2005283. [DOI] [PubMed] [Google Scholar]