Abstract
Background
Hepatocellular carcinoma, also called malignant hepatoma, is a primary malignancy of the liver. Despite regular surveillance conducted in high‐risk populations, most people with hepatocellular carcinoma are diagnosed at an advanced stage. Consequently, only a minority of people with the disease are suitable for surgical resection when diagnosed.
Objectives
To compare the beneficial and harmful effects of transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone in adults with primary hepatocellular carcinoma, considered unsuitable for surgical resection.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, LILACS, Science Citation Index Expanded, and Conference Proceedings Citation Index – Science up to 31 May 2018. We checked reference lists for all included studies and related reviews for further relevant articles.
Selection criteria
We included all randomised clinical trials comparing TACE followed by 3‐DCRT versus TACE alone in people with primary hepatocellular carcinoma.
Data collection and analysis
We used standard methodological procedures as suggested by Cochrane. We presented the results of the fixed‐effect model in the absence of statistical heterogeneity. Otherwise, we reported the results from the random‐effects model meta‐analysis. We assessed risk of bias of the included trials using bias risk domains and presented the review results incorporating the methodological quality of the trials using GRADE. Our main conclusions were based on the analysis up to three years' follow‐up.
Main results
We identified eight randomised clinical trials (632 participants) that fulfilled our inclusion criteria. All eight trials were at high risk of bias, and we rated the evidence as low to very low certainty. The mean age ranged from 16 years to 78 years. The proportion of men ranged from 60% to 75% and the proportion of people with stage III primary hepatocellular carcinoma ranged from 22% to 85%. The median follow‐up duration was 12 months (2 months to 38 months).
TACE followed by 3‐DCRT compared with TACE alone may have reduced all‐cause mortality at three years' follow‐up (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.73 to 0.88; 552 participants; 7 trials; low‐certainty evidence). TACE followed by 3‐DCRT compared with TACE alone may reduce the proportion of participants without tumour response (complete response plus partial response) (RR 0.49, 95% CI 0.39 to 0.61; 632 participants; 8 trials; low‐certainty evidence). Data, from one trial on health‐related quality of life, favoured the TACE followed by 3‐DCRT group, but the provided data were ill‐defined (very low‐certainty evidence). None of the trials reported serious adverse events. The results on non‐serious adverse events were as follows: TACE followed by 3‐DCRT compared with TACE alone showed no difference in the results for proportion of participants with leukopenia (RR 1.12, 95% CI 0.92 to 1.34; 438 participants; 5 trials; very low‐certainty evidence) and serum transaminases elevation (RR 1.67, 95% CI 0.66 to 4.27; 280 participants; 4 trials; very low‐certainty evidence). However, the proportion of participants with total bilirubin elevation was larger in the TACE followed by 3‐DCRT group than in the TACE alone group (RR 2.69, 95% CI 1.34 to 5.40; 172 participants; 2 trials; very low‐certainty evidence). The rate of participants with serum alpha‐fetoprotein (AFP) without decline or normalisation was significantly lower in the TACE followed by 3‐DCRT group than in the TACE group, but these data were from one trial only (Chi² = 7.24, P = 0.007; very low‐certainty evidence).
Authors' conclusions
TACE followed by 3‐DCRT may be associated with lower all‐cause mortality and increased tumour response, despite the increased toxicity expressed by a higher rise of total bilirubin. Our review findings should be considered with caution because of the methodological weaknesses in the included trials, resulting in low‐ to very low‐certainty evidence. Data on serious adverse events and health‐related quality of life are lacking. We are also very much uncertain in the results of the reported non‐serious adverse events. High‐quality trials are needed to assess further the role of TACE followed by 3‐DCRT for unresectable hepatocellular carcinoma.
Plain language summary
Transcatheter arterial chemoembolisation followed by three‐dimensional conformal radiotherapy for primary hepatocellular carcinoma
Background
Hepatocellular carcinoma, also called malignant hepatoma, is a primary liver cancer. Despite regular surveillance conducted in high‐risk populations, most people with hepatocellular carcinoma are diagnosed at an advanced stage. Consequently, a minority of the people with the disease are suitable for surgical resection (removal). Since transcatheter arterial chemoembolisation (TACE; a procedure to restrict the blood supply to a tumour) was introduced as a palliative (to relieve symptoms and improve quality of life) treatment in people with unresectable liver cancer, it has become one of the most common forms of intervention. More recently, the modern radiation technology of three‐dimensional conformal radiotherapy (3‐DCRT), which shapes the radiation beams to the shape of the tumour, has been used to improve the adverse effects of conventional radiotherapy. It is predicted that the combination of TACE followed by 3‐DCRT could enhance the treatment effect for hepatocellular carcinoma. To date, little is known about the benefits and harms of the combination of TACE followed by 3‐DCRT, and current studies are still controversial for the efficacy of the combination of TACE followed by 3‐DCRT compared with TACE alone. The aim of this Cochrane systematic review was to compare the benefits and harms of TACE followed by 3‐DCRT versus TACE alone in people with primary hepatocellular carcinoma, considered to be unsuitable for surgical removal.
Study characteristics
The review authors searched the medical literature in order to clarify the role of the combination of TACE followed by 3‐DCRT for the treatment of primary hepatocellular carcinoma, and to compare their benefits and harms with TACE alone. We collected and analysed data from randomised clinical trials (clinical studies where people are randomly put into one of two or more treatment groups) of people with primary hepatocellular carcinoma who were able to receive TACE or 3‐DCRT. Evidence is current to May 2018.
Key results and quality of evidence
The review included eight trials with 632 participants. All trials were at high risk of bias. TACE followed by 3‐DCRT appeared to be superior to TACE in improving death from any cause and tumour response, despite an increased toxicity expressed by a higher rise of total bilirubin (measured by a blood test to see how well the liver is working). No trials reported serious side effects. One trial reported health‐related quality of life (a measure of a person's satisfaction with their life and health), but this was ill‐defined. The review findings were uncertain because the included trials had methodological weaknesses. More high‐quality randomised clinical trials are needed to confirm or complete the review findings.
Summary of findings
Summary of findings for the main comparison. TACE followed by 3‐DCRT compared to TACE for primary hepatocellular carcinoma.
| TACE followed by 3‐DCRT compared to TACE for primary hepatocellular carcinoma | ||||||
|
Patient or population: primary hepatocellular carcinoma Setting: hospitalised in China Intervention: TACE followed by 3‐DCRT Comparison: TACE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with TACE | Risk with TACE+3‐DCRT | |||||
|
All‐cause mortality: at 3 years Follow‐up: mean 17 months |
Study population | RR 0.80 (0.73 to 0.88) | 552 (7 RCTs) | ⊕⊕⊝⊝ Lowa | — | |
| 853 per 1000 | 683 per 1000 (623 to 751) | |||||
|
Proportion of participants without tumour response (CR+PR) Follow‐up: mean 18 months |
Study population | RR 0.49 (0.39 to 0.61) | 632 (8 RCTs) | ⊕⊕⊝⊝ Lowa | — | |
| 495 per 1000 | 243 per 1000 (193 to 302) | |||||
| Serious adverse events | None of the trials reported data on serious adverse events. | ⊕⊝⊝⊝ Very lowa,b | — | |||
| Health‐related quality of life | Health‐related quality of life was significantly better in the TACE followed by 3‐DCRT group than in the TACE alone group (Chi² = 4.479, P = 0.034) | 66 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | ||
|
Non‐serious adverse events: leukopenia Follow‐up: mean 13.2 months |
Study population | RR 1.12 (0.92 to 1.34) | 438 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,c | — | |
| 475 per 1000 | 532 per 1000 (437 to 636) | |||||
|
Non‐serious adverse events: serum transaminases elevation Follow‐up: mean 7.5 month |
Study population | RR 1.67 (0.66 to 4.27) | 280 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,d,e,f | — | |
| 328 per 1000 | 549 per 1000 (217 to 1000) | |||||
|
Non‐serious adverse events: total bilirubin elevation Follow‐up: mean 6 months |
Study population | RR 2.69 (1.34 to 5.40) | 172 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,g | — | |
| 108 per 1000 | 292 per 1000 (145 to 586) | |||||
| Proportion of participants without serum AFP normalisation | The rate of participants with serum AFP without decline or normalisation was a significantly lower in the TACE followed by 3‐DCRT group than in the TACE alone group (Chi² = 7.24, P = 0.007) | 96 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 3‐DCRT: three‐dimensional conformal radiotherapy; AFP: alpha fetoprotein; CI: confidence interval; CR: complete response; PR: partial response; RCT: randomised clinical trial; RR: risk ratio; TACE: transcatheter arterial chemoembolisation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for risk of bias: most of the included RCTs had unclear risk of concealment of allocation, non‐blinded assessment of outcomes, attrition bias, and other bias. bDowngraded one level for imprecision: we were unable to combine the data in an overall analysis due to lack of data. cDowngraded one level for heterogeneity: the heterogeneity test showed that variation existed in point estimates due to among‐study differences. dDowngraded one level for heterogeneity: the heterogeneity test showed that large variation (I² = 88%) existed in point estimates due to among‐study differences. eDowngraded two levels for imprecision: the sample size was less than 300 (280 participants), the number of events not high (108 events), and the confidence intervals of the pooled RR clearly crossed the line of no effect and appreciable harm. fDowngraded one level for publication bias; based on the funnel plot. gDowngraded one level for imprecision: the sample size was less than 300 (172 participants).
Background
Description of the condition
Hepatocellular carcinoma, also called malignant hepatoma, is a primary malignancy of the liver. It is the fifth most common neoplasm worldwide and its incidence is increasing (Venook 2010; Ferlay 2015). It is the second leading cause of cancer‐related death (El‐Serag 2014). Most people with hepatocellular carcinoma develop malignancy secondary to either viral hepatitis infections (hepatitis B or hepatitis C) or cirrhosis (alcoholism being the most common cause of hepatic cirrhosis) (Kumar 2004).
Hepatocellular carcinoma exhibits two main global patterns: one in North America and Western Europe where the prevalence of chronic hepatitis C virus is increasing, and another in non‐Western countries, such as those in sub‐Saharan Africa, Central and Southeast Asia, and the Amazon basin where the prevalence of chronic hepatitis B is high (El‐Serag 2001; Lodato 2006). Epidemiological data show that the incidence of hepatocellular carcinoma is changing around the world following aetiology; it is increasing in many high‐income countries, whereas it is declining in low‐income countries (McGlynn 2001). Usually, there are more men than women who develop hepatocellular carcinoma, and the women are usually between 30 years and 50 years of age (Kumar 2004). Yearly, 610,000 people die of hepatocellular carcinoma worldwide (WHO 2009), and about half of these deaths occur in China. Hepatocellular carcinoma is one of the deadliest cancers in China, where chronic hepatitis B is found to be the cause in 90% of the deaths. With the introduction of hepatitis B virus vaccination, the incidence of hepatocellular carcinoma has been decreasing in low‐income countries (Lodato 2006). In Japan, 90% of hepatocellular carcinomas are associated with hepatitis C. Food infected with Aspergillus flavus (especially peanuts and corns stored during prolonged wet seasons), which produces aflatoxin, poses another risk factor for hepatocellular carcinoma. However, most malignant tumours of the liver discovered in people from Western countries are metastases from tumours elsewhere, and hepatocellular carcinoma is generally seen as a rare cancer (Lodato 2006). Hepatocellular carcinoma is one of the few types of cancer that has increased in frequency and mortality in the USA (Mittal 2013) and Europe (Deuffic 1998).
Hepatocellular carcinoma at its early stages is often non‐symptomatic. As the cancer grows, symptoms may include pain in the upper abdomen on the right side, which may extend to the back and shoulder, or cause swollen abdomen (bloating), weight loss, loss of appetite, loss of the sensation of being full, fatigue, nausea and vomiting, jaundice, or fever. Mostly, these symptoms happen in stages III or IV of the disease. Diagnosis of hepatocellular carcinoma may involve physical examination such as examination of the liver, spleen, or any lumps; ascites; and jaundice. It may also entail an alpha‐fetoprotein (AFP) test, computer tomography scan, ultrasound, magnetic resonance imaging, angiogram, or biopsy (El‐Serag 2008). Despite regular surveillance conducted in high‐risk populations, most people with hepatocellular carcinoma are diagnosed at an advanced stage. Consequently, a minority of people with the disease are suitable for surgical resection. The recurrence rates are as high as 65% to 80% within five years, even for those people who undergo surgical resection (Li 2013), which results in a five‐year survival of about 40% (Cha 2005).
Description of the intervention
People with early‐stage cancer (20% to 30% of people with hepatocellular carcinoma) are considered suitable for treatments such as liver resection, liver transplantation, percutaneous ablation, percutaneous ethanol injection, and radiofrequency ablation (Llovet 2004; Cabrera 2010). Since transcatheter arterial chemoembolisation (TACE) was introduced as a palliative treatment in people with unresectable hepatocellular carcinoma, it has become one of the most common forms of intervention (Takayasu 2006); and it is considered a standard treatment option for people with unresectable hepatocellular carcinoma (Kothary 2007). In addition, TACE is usually performed as a temporary treatment while waiting for a liver transplant, or for people for whom surgical or percutaneous ablative treatment is contraindicated. TACE involves the injection of anticancer drugs (doxorubicin, epirubicin, or cisplatin) and iodised oil (Lipiodol Ultra‐Fluide, Laboratoires Guerber, Aulnay‐sous‐Bois, France) (¹³¹I‐lipiodol radiotherapy) into the hepatic artery, followed by the administration of embolic agents (Nakamura 1990; Bronowicki 1994). Currently, TACE is considered the standard of care for people with intermediate‐stage hepatocellular carcinoma presenting with Child‐Pugh class A and B liver function, and large or multinodular hepatocellular carcinoma without cancer‐related symptoms, macrovascular invasion, or extrahepatic metastasis (Murata 2014).
These treatment recommendations occur irrespective of the fact that a Cochrane systematic review has been unable to identify high‐quality evidence in support of TACE (Oliveri 2011).
Since the early 2000s, the modern radiation technology of three‐dimensional conformal radiotherapy (3‐DCRT) has been applied in clinics to improve the shortcomings of conventional radiotherapy. Radiotherapy for hepatocellular carcinoma has resulted in unsatisfactory outcomes since the late 1980s because of the liver's poor tolerance to irradiation (Liang 2005). 3‐DCRT is aided by a computerised treatment‐planning system which has enabled the tight conformation of a high‐dose volume to hepatocellular carcinoma lesions in three dimensions. Thus, 3‐DCRT has made it possible to escalate the irradiation dose to focal hepatocellular carcinoma without causing undue dose‐limiting toxicity in neighbouring non‐cancerous liver tissues. Therefore, it spares non‐cancerous liver tissue from excess damage, and has increasingly been recognised as a potentially curative option for people with hepatocellular carcinoma (Lawrence 1990; Feng 2011).
How the intervention might work
TACE is appropriate for hepatocellular carcinoma, as the hepatic artery delivers 99% of the blood supply to hepatic tumours (Murata 2014). TACE seems to improve survival compared with the best supportive care in meta‐analyses of randomised trials (Cammà 2002; Llovet 2003); and in two individual clinical trials (Llovet 2002; Lo 2002). The antitumour effect of TACE is greater than that of other anticancer drugs (Yoshikawa 1994); or iodised oil alone (Takayasu 1987; Yamagami 2014). As stated above, we lack high‐quality evidence in support of TACE (Oliveri 2011).
With advances in 3‐DCRT, local radiation of the liver has become safer (Robertson 1993); and its efficacy is better than in conventional radiotherapy (Matsuura 1998). 3‐DCRT has shown favourable outcomes in local control and survival, with a median survival time of 10 months to 25 months (Yu 2014); and a three‐year survival of around 30% for people with hepatocellular carcinoma (Lee 2013). Several series that employed 3‐DCRT have reported a dose‐response relation in radiotherapy for liver cancers with better response rates and prolonged hepatic control in groups that received higher radiotherapy doses (Robertson 1993; Seong 2000).
The inadequacy of single TACE in inducing complete tumour necrosis has also been well documented (Sasaki 1987), and TACE is usually repeated at regular intervals. Nevertheless, repeated TACE frequently becomes ineffective due to tumour progression. As most primary liver tumours have dual blood supplies, it is easy to re‐form the collateral circulation in lesions with the residual tumour cells after TACE (Ikeda 1991; Cheng 2000). It is predicted that the combination of TACE and 3‐DCRT could enhance treatment effects for hepatocellular carcinoma. It was reported that the one‐year survival rate for the combination of TACE followed by 3‐DCRT was 73%, two‐year survival rate was 53%, and three‐year survival rate was 35%, which was higher than the TACE alone group (one‐year survival rate 60%, two‐year survival rate 31%, and three‐year survival rate 14%) (Zou 2014).
The rationale for combined TACE and 3‐DCRT was based on the following three considerations. First, with the iodised oil injection by TACE, the deposit of iodine would have made the margin of the median gross tumour volume clearer, making gross tumour volume delineation more accurate, and 3‐DCRT plan verification easier. Second, after TACE, the tumour burden becomes less and the number of tumour cells decreases. This would make it easier for 3‐DCRT to control the malignancy. Third, the 3‐DCRT radiosensitivity of hepatocellular carcinoma still exists when 3‐DCRT begins several weeks after TACE (Zhou 2007).
Why it is important to do this review
To date, little is known about the benefits and harms of the combination of TACE and 3‐DCRT, and only few clinical studies have been conducted. Some were in favour of the combination of TACE and 3‐DCRT (Shim 2005; Koo 2010), while another showed that the survival rates of people with combined TACE and 3‐DCRT were similar to those with TACE alone (Chia‐Hsien 2001). Despite the publication of further studies on the use of TACE followed by 3‐DCRT in people with primary hepatocellular carcinoma, we found no systematic reviews or meta‐analyses with randomised clinical trials comparing the combination of TACE followed by 3‐DCRT versus TACE alone.
Objectives
To compare the beneficial and harmful effects of transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone in adults with primary hepatocellular carcinoma, considered unsuitable for surgical removal.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised clinical trials investigating a combination of TACE and 3‐DCRT versus TACE alone for inclusion, whether they were double‐blind, single‐blind, or open‐label, and regardless of publication status, language, and length of the trial. In addition, we scanned quasi‐randomised and other observational studies which were retrieved with the searches for randomised clinical trials to identify reports on harm. By not searching specifically for harms in observational studies, we are aware that we, in the present systematic review, may have been biased towards assessing benefits and ignoring harms (see Storebø 2018).
Types of participants
Participants older than 18 years diagnosed with hepatocellular carcinoma based on their pathological findings in at least one lesion with or without other laboratory evidence such as B‐ultrasound, computed tomography, or AFP. Furthermore, diagnosis had to conform to the following criteria.
Participants had not received any anticancer therapy.
Karnofsky score was 69 or less (Park 2014).
Child‐Pugh grade of liver function was A or B (Liang 2015).
Number of white blood cell 4.0 × 10⁹/L or greater.
Model for end‐stage liver disease (MELD) score less than 10.
There were no contraindications (vascular or adjacent organ involvement, involvement with lymph nodes, distant metastasis, jaundice and ascites, cardiopulmonary dysfunction, coagulation disorders) of TACE and 3‐DCRT.
Types of interventions
We included trials comparing TACE followed by 3‐DCRT versus TACE alone in people with primary hepatocellular carcinoma.
Types of outcome measures
We sought to measure the following outcomes at the end of treatment, as well as at maximal follow‐up.
Primary outcomes
All‐cause mortality (death from any cause). We calculated one‐year, two‐year, and three‐year all‐cause mortality. We drew primary conclusions based on three‐year all‐cause mortality, as the longer the follow‐up period, the stronger the evidence.
-
Proportion of participants without tumour response: according to the World Health Organization (WHO) Handbook for reporting the results of cancer treatment (Spieth 2003), the responses were assessed as follows:
complete response (CR), complete disappearance or 100% necrosis of all tumours with no evidence of new lesions;
partial response (PR), more than 50% reduction or more than 50% necrosis (or both) of all measurable lesions with no evidence of new lesions;
progressive disease (PD), more than 25% enlargement of all measurable lesions or appearance of new lesions;
stable disease (SD), no change.
Serious adverse events: we used the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice's definition of a serious adverse event (ICH‐GCP 1997); that was, any untoward medical occurrence that resulted in death, was life threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was a congenital anomaly or birth defect. We considered all other adverse events as non‐serious.
Secondary outcomes
Health‐related quality of life as reported in the trials.
Non‐serious adverse events, such as abdominal pain, fatigue, poor appetite, nausea, vomiting, fever, leukopenia, thrombocytopenia, MELD score, etc. We analysed the following non‐serious adverse events separately: proportion of participants with leukopenia, with serum transaminases elevation, and with total bilirubin elevation.
Proportion of participants without serum AFP normalisation.
Search methods for identification of studies
Electronic searches
We searched the The Cochrane Hepato‐Biliary Group Controlled Trials Register (May 2018; Cochrane Hepato‐Biliary Group Module), the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2018, Issue 4), MEDLINE Ovid (1946 to May 2018), Embase Ovid (1974 to May 2018), LILACS (1982 to May 2018; Bireme), Science Citation Index Expanded (1900 to May 2018; Web of Science), and Conference Proceedings Citation Index – Science (1990 to May 2018; Web of Science) (Royle 2003). We checked reference lists of all included studies and related reviews manually for further related articles. Appendix 1 provided the search strategies with the time spans of the searches.
Searching other resources
We searched the reference lists of the identified trials to identify further relevant trials.
We also searched online trial registries such as ClinicalTrials.gov (clinicaltrials.gov/), European Medicines Agency (EMA; www.ema.europa.eu/ema/), WHO International Clinical Trials Registry Platform (www.who.int/ictrp), and the Food and Drug Administration (FDA; www.fda.gov), as well as pharmaceutical company sources, for ongoing or unpublished trials.
Data collection and analysis
We performed the review according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module. We used the Cochrane statistical software Review Manager 5, for data entry and analysis (Review Manager 2014).
Selection of studies
Two review authors (LL and JZ) independently identified the trials for inclusion. We listed the excluded studies with their reasons for exclusion. We resolved disagreements by discussion with the other author (WZ). We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review.
Data extraction and management
Two review authors (LL and JZ) independently extracted the following data from each trial.
Year and language of publication.
Country.
Year trial was conducted.
Inclusion and exclusion criteria.
Sample size.
Population characteristics such as age, sex ratio, Karnofsky score and Child‐Pugh grade of liver function.
For TACE, use of drugs for chemotherapy and embolisation.
For 3‐DCRT, use of irradiation such as dosage, frequency, and range.
Treatment measures for adverse effects.
Outcomes (see Primary outcomes; Secondary outcomes).
Methodological quality and bias risk.
Sample size calculation.
Intention‐to‐treat (ITT) analysis.
We sought missing information or clarification of unclear information by contacting the authors of the individual trials. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
Two review authors (LL; JZ) independently assessed the risk of bias for each included trial according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the Cochrane Hepato‐Biliary Group Module, and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017; Savović 2018).
We used the following definitions in the assessment of risk of bias.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, or throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: method of sequence generation was not specified.
High risk of bias: sequence generation method was not random.
Allocation concealment
Low risk of bias: participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. Allocation sequence was unknown to the investigators (e.g. whether the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: allocation sequence was likely known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judged this outcome unlikely to have been influenced by lack of blinding (mortality) (Wood 2008; Savović 2012a; Savović 2012b); or blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to have been influenced; or blinding of key study participants and personnel was attempted, but likely could have been broken, thus influencing the outcome.
Blinding of outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judged that the outcome measurement was unlikely to have been influenced by a lack of blinding; or blinding of outcome assessment was ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to have been influenced by a lack of blinding; or the outcome assessment was blinded, but it was likely that the blinding could have been broken, and the outcome measurement was therefore likely to have been influenced.
Incomplete outcome data
Low risk of bias: missing data were unlikely to have made treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data, in combination with the method used to handle missing data, were likely to have induced bias in the results.
High risk of bias: the results were likely to have been biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, tumour response assessments, and serious adverse events. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. clinicaltrials.gov/), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time it began. If the trial protocol was registered after the trial began, those outcomes were not considered reliable.
Unclear risk of bias: not all predefined outcomes were reported in full, or it was unclear whether data on these outcomes had been recorded or not.
High risk of bias: one or more predefined outcomes was not reported.
Other bias
Low risk of bias: the trial appeared to be free of other bias that could put its integrity at risk.
Unclear risk of bias: the trial may or may not have been free of other domains that could have put it at risk of bias.
High risk of bias: there were other factors in the trial that could have put it at risk of bias.
We judged trials to be at an overall low risk of bias if assessed with a low risk of bias in all above domains. We judged trials to be at an overall high risk of bias if assessed with unclear risk of bias or high risk of bias in one or more of the above domains.
Measures of treatment effect
We performed the meta‐analyses according to Cochrane recommendations (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module. We used the Review Manger 5 software package provided by Cochrane (Review Manager 2014). For dichotomous variables, we calculated the risk ratio (RR) with a 95% confidence interval (CI). For continuous variables, we calculated the mean difference with a 95% CI.
Unit of analysis issues
We took into account the group of participants per intervention group in the randomised clinical trials with parallel‐group design. In the case of cross‐over trials, we planned to use the data from the first trial period only. We did not expect to find cluster‐randomised trials. For trials with multiple intervention groups, we planned to include the groups in which our experimental and control interventions were compared. We planned to divide the control group into two or more to avoid double‐counting in case it was a common comparator.
Dealing with missing data
We considered participants with completely missing data as treatment failures and performed ITT analyses. If data for any participant were obtained at any point before the measured time point, this observation was carried forward.
Assessment of heterogeneity
We explored heterogeneity using the Chi² test with significance set at a P value of 0.10 or less, and measured the extent of heterogeneity using the I² statistic (Higgins 2002). We interpreted I² values as follows:
probably not important: 0% to 40%;
possible moderate heterogeneity: 30% to 60%;
possible substantial heterogeneity: 50% to 90%;
considerable heterogeneity: 75% to 100%.
Assessment of reporting biases
We used visual asymmetry on a funnel plot to explore reporting bias when at least 10 randomised clinical trials were identified in a particular field (Egger 1997; Macaskill 2001). In addition, we performed the linear regression approach described by Egger to determine the funnel plot asymmetry if the result of a funnel plot was unclear (Egger 1997).
Data synthesis
Meta‐analysis
We performed our meta‐analyses in accordance with Cochrane's recommendations (Higgins 2011), and used Review Manager 5 software for our analyses (Review Manager 2014). We evaluated all missing data using ITT analyses. We treated missing data as treatment failures.
We expressed binary outcomes using RR with 95% CI. If the results were statistically significant according to our Trial Sequential Analysis (see 'Trial Sequential Analysis' below), we calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) using fixed‐effect and random‐effects models (DerSimonian 1986; DeMets 1987). We interpreted the results according to Jakobsen 2014. If there is absence of statistical heterogeneity or only one trial was included, the fixed‐effect and the random‐effects models would show identical results. We presented the results of the fixed‐effect model in this situation. If there was substantial statistical heterogeneity, we reported the results from the random‐effects meta‐analysis. We presented unavailable data and inappropriate data using descriptive means.
Trial Sequential Analysis
We applied Trial Sequential Analysis as cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). To minimise random errors, we calculated the required information size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008). We calculated the required information size adjusted for diversity, since the heterogeneity adjustment with the I² statistic underestimated the required information size (Wetterslev 2008; Wetterslev 2009). In our meta‐analysis, we performed Trial Sequential Analysis to maintain an overall 2.5% risk of a type I error and 20% of type II error (a power of 80%) (Wetterslev 2009). On the basis of the required information size, we constructed trial sequential monitoring boundaries (Lan 1983; Wetterslev 2008; Thorlund 2011). These boundaries determined the statistical inference one may draw regarding the cumulative meta‐analysis that had not reached the required information size; if the trial sequential monitoring boundary for benefit or harm was crossed before the required information size was reached, firm evidence may have been established, and further trials may have turned out to be superfluous. In contrast, if the boundary had not been surpassed, it was probably necessary to continue doing trials in order to detect or reject a certain intervention effect. This could be determined by assessing whether the cumulative Z‐curve crossed the trial sequential boundaries for futility. If futility boundaries had been crossed, then further trials may have been unnecessary (TSA 2011). We conducted Trial Sequential Analysis using software from The Copenhagen Trial Unit (Thorlund 2011; TSA 2011; Wetterslev 2017).
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses.
Trials at low bias risk compared to trials at high bias risk.
With TACE, different drugs used in chemotherapy and embolisation.
With 3‐DCRT, different dosage, frequency, and range in irradiation.
Presence or absence of chronic liver disease.
Aetiology of the chronic liver disease.
Sensitivity analysis
We assessed the robustness of our analyses by performing a sensitivity analysis, excluding studies from the overall analysis of high risk of bias due to lack of allocation concealment, blinding, or incomplete reporting of primary outcome. We compared the GRADE assessment of imprecision with that obtained with Trial Sequential Analysis (Jakobsen 2014; Castellini 2018).
'Summary of findings' tables
We presented the evidence in Table 1 using GRADEpro software in accordance with the principles of the GRADE system (Guyatt 2011a). This was done to assess the certainty of the body of evidence associated with specific outcomes such as all‐cause mortality, recent objective response of hepatocellular carcinoma, and serious adverse events in our review.
The GRADE approach defined the certainty in a body of evidence as the extent to which one could be confident that an estimate of effect or association was close to the quantity of specific interest. According to GRADE, the certainty in a body of evidence included five factors regarding limitations in the design and implementation of available studies suggesting high likelihood of bias: indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results (wide CIs); and high probability of publication bias (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Mustafa 2013; Guyatt 2017).
Results
Description of studies
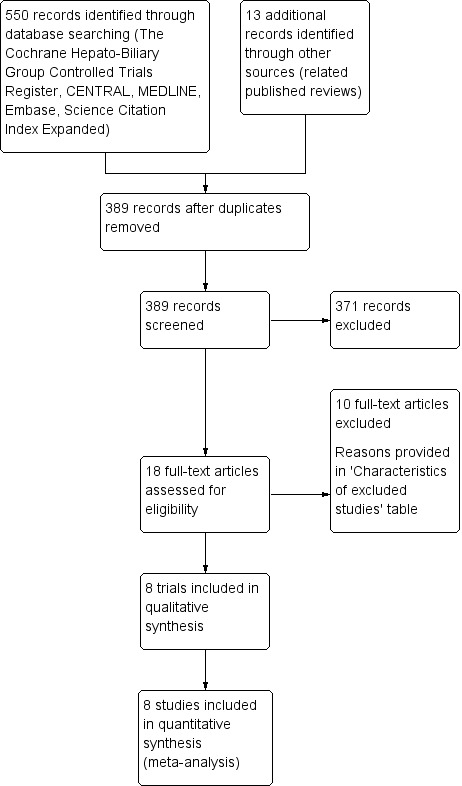
The search identified 550 reports (Figure 1).
1.
Study flow diagram.
Results of the search
Electronic literature searches revealed two hits in The Cochrane Hepato‐Biliary Group Controlled Trials Register, 12 hits in the Cochrane Central Register of Controlled Trials (CENTRAL), 104 hits in MEDLINE, 212 hits in Embase, zero hits in LILACS, and 220 hits in Science Citation Index Expanded and Conference Proceedings Citation Index – Science. Appendix 1 shows the search strategies. Thirteen reports consisted of additional reports on published reviews (Zou 2014; Bai 2016). After removing duplicates, 389 reports remained. We excluded 371 irrelevant reports based on the title, abstract, or both. We retrieved and read the full‐text of 18 reports, and finally included eight trials (eight reports).
Included studies
Eight trials satisfied our inclusion criteria (Zhao 2006; Shang 2007; Xiao 2008; Ning 2009; Liao 2010; Gong 2011; Xiao 2011; Chen 2014).
Characteristics of included studies
We summarised the characteristics of the eight included trials in the Characteristics of included studies table.
Study design
All trials were parallel group randomised clinical trials.
Funding
Only one trial was supported by a grant from the local science and technology bureau (Liao 2010). Other trials did not provide any data on funding.
Participants
There were 632 participants with primary hepatocellular carcinoma. The mean age ranged from 16 years to 78 years. The proportion of men ranged from 60% to 75%, and the proportion of people with stage III primary hepatocellular carcinoma ranged from 22% to 85%. The proportion of people with tumour size greater than 10 cm was 45% in one trial (Chen 2014), and with greater than 3 cm size ranging from 40% to 100% in five trials (Zhao 2006; Shang 2007; Xiao 2008; Liao 2010; Xiao 2011). The proportion of people with a single tumour was 68% to 75% in two trials (Xiao 2011; Chen 2014), the proportion with Child‐Pugh class A ranged from 65% to 71% in two trials (Xiao 2008; Liao 2010), and the proportion with AFP greater than 400 μg/L ranged from 41% to 100% in three trials (Zhao 2006; Shang 2007; Xiao 2011). Accordingly, these participants would mostly likely be considered unsuitable for surgical resection.
Interventions
People underwent two courses (Zhao 2006; Shang 2007; Xiao 2008; Ning 2009; Gong 2011; Xiao 2011; Chen 2014) or three to five courses (Liao 2010) of TACE with one‐month interval. 3‐DCRT was delivered one to four weeks after the last course of TACE, if liver function tests were normal. The sum of the radiation doses in 3‐DCRT received by each individual ranged from 30 Gray (Gy) to 66 Gy with 2 Gy/day to 5 Gy/day and 3 days/week to 5 days/week.
Comparisons
All eight trials compared TACE followed by 3‐DCRT versus TACE alone. The chemotherapy included 5‐fluorouracil (750 mg to 1250 mg) (Zhao 2006; Shang 2007; Xiao 2008; Ning 2009; Liao 2010; Gong 2011; Chen 2014), cisplatin (40 mg to 120 mg) (Zhao 2006; Shang 2007; Xiao 2008; Ning 2009; Liao 2010; Gong 2011; Xiao 2011; Chen 2014), adriamycin (30 mg to 100 mg) (Shang 2007; Xiao 2008; Liao 2010; Xiao 2011), hydroxyl radical (15 mg to 20 mg) (Zhao 2006; Ning 2009), and mitomycin C (6 mg to 14 mg) (Ning 2009; Chen 2014). Embolisation therapy: peripheral embolisation was performed by iodine oil emulsion, and central embolisation was performed by gelfoam.
Outcomes
All trials reported all‐cause mortality and tumour response. Duration of therapy and embolisation were similar in the two intervention groups. No trial reported serious adverse events. Only one trial reported the rate of participants without decline or normalisation of AFP (Zhao 2006), and another trial provided some information on health‐related quality of life (Ning 2009). Five trials reported non‐serious adverse events (Zhao 2006; Shang 2007; Xiao 2008; Liao 2010; Chen 2014).
Excluded studies
See Characteristics of excluded studies table.
We excluded 10 observational studies (Chia‐Hsien 2001; Zeng 2004; Guo 2005; Liang 2005; Shim 2005; Chung 2006; You 2007; Zhang 2009; Koo 2010; Lu 2015).
Risk of bias in included studies
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
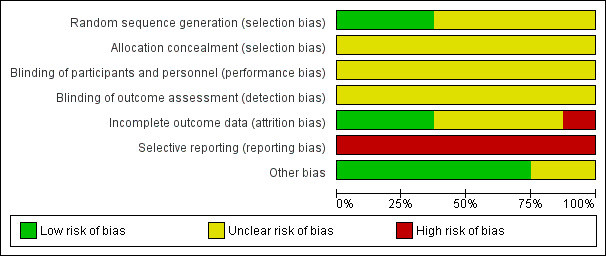
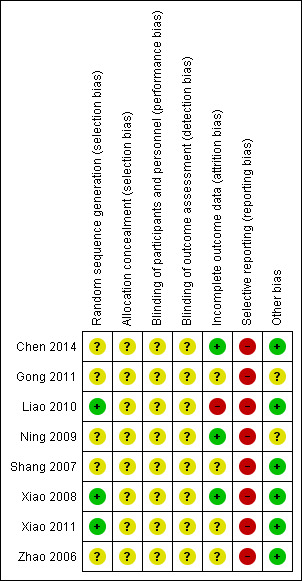
All trials were at high risk of bias (Zhao 2006; Shang 2007; Xiao 2008; Ning 2009; Liao 2010; Gong 2011; Xiao 2011; Chen 2014).
Allocation
Three trials performed random sequence generation using a random number table, and hence were at low risk of bias (Xiao 2008; Liao 2010; Xiao 2011). The remaining five studies had unclear risk of bias, falling into the group of high risk of bias trials.
Allocation concealment had unclear risk of bias in all trials, as the trials provided no information.
Blinding
All trials were at unclear risk of bias for blinding of participants and investigators. Also, detection bias was unclear in all studies, hence all trials were at high overall risk of bias.
Incomplete outcome data
Three trials were at low risk of attrition bias, as they did not have any missing data after randomisation (Xiao 2008; Ning 2009; Chen 2014). One trial had high risk of bias because it did not account for participants with missing outcomes (Liao 2010). Other trials were at unclear risk of bias (Zhao 2006; Shang 2007; Gong 2011; Xiao 2011).
Selective reporting
All trials were at high risk of bias for selective reporting as none reported one clinically relevant outcome (serious adverse events); not all protocols were available.
Other potential sources of bias
We assessed six trials as having a low risk of bias regarding other potential sources of bias such as demographic and baseline characteristics of the randomised participants (Zhao 2006; Shang 2007; Xiao 2008; Liao 2010; Xiao 2011; Chen 2014), and we considered the remaining two trials as having unclear risk of bias because the trial authors did not provide the demographic and baseline characteristics of the randomised participants (Ning 2009; Gong 2011).
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
All‐cause mortality
At one‐year, 85/319 (26.6%) participants treated with TACE followed by 3‐DCRT and 155/313 (49.5%) participants treated with TACE died. There was a lower end of one‐year all‐cause mortality rate in the TACE followed by 3‐DCRT group than in the TACE group (RR 0.54, 95% CI 0.44 to 0.66; 632 participants; 8 trials; Analysis 1.1). There was no trial heterogeneity (Chi² = 7.00, P = 0.43; I² = 0%).
1.1. Analysis.
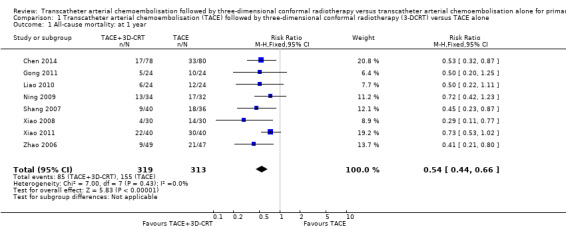
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 1 All‐cause mortality: at 1 year.
By the end of the second year, all randomised clinical trials, except for Liao 2010, provided mortality data: 143/295 (48.5%) participants treated with TACE followed by 3‐DCRT and 205/289 (70.9%) participants treated with TACE alone died (RR 0.68, 95% CI 0.60 to 0.78; 584 participants; 7 trials; Analysis 1.2). There was no trial heterogeneity (Chi² = 5.46, P = 0.49; I² = 0%).
1.2. Analysis.
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 2 All‐cause mortality: at 2 years.
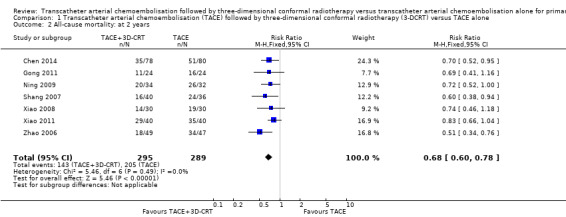
By the end of the third year, all randomised clinical trials, except for Xiao 2011, provided mortality data: 191/279 (68.5%) participants treated with TACE followed by 3‐DCRT and 233/273 (85.3%) participants treated with TACE died. (RR 0.80, 95% CI 0.73 to 0.88; 552 participants; 7 trials; Analysis 1.3). There was no trial heterogeneity (Chi² = 4.08, P = 0.67; I² = 0%).
1.3. Analysis.
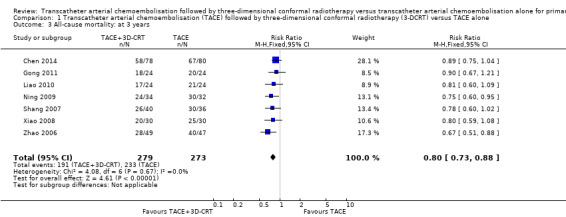
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 3 All‐cause mortality: at 3 years.
Proportion of participants without tumour response (complete and partial)
All randomised clinical trials compared TACE followed by 3‐DCRT versus TACE alone in participants without tumour response: 77/319 (24.1%) participants treated with TACE followed by 3‐DCRT and 155/313 (49.5%) participants treated with TACE alone remained without tumour response (RR 0.49, 95% CI 0.39 to 0.61; 632 participants; 8 trials; Analysis 1.4). There was no trial heterogeneity (Chi² = 2.06, P = 0.96; I² = 0%).
1.4. Analysis.
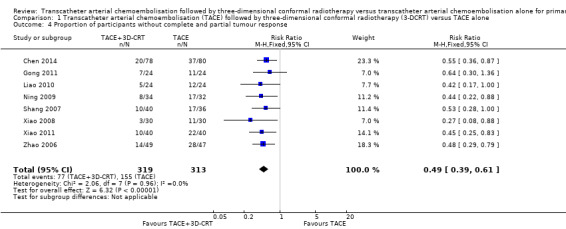
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 4 Proportion of participants without complete and partial tumour response.
Serious adverse events
No trials reported serious adverse events.
Secondary outcomes
Health‐related quality of life
We could not perform an analysis of health‐related quality of life as the information reported in the randomised clinical trials was insufficient. Only one trial reported scant data (Ning 2009). Health‐related quality of life was significantly better in the TACE followed by 3‐DCRT group than in the TACE group (Chi² = 4.479, P = 0.034).
Non‐serious adverse events
There was no significant difference in the TACE followed by 3‐DCRT group compared with the TACE alone group regarding the proportion of trial participants with leukopenia (RR 1.12, 95% CI 0.92 to 1.34; 438 participants; 5 studies; I² = 67%; Analysis 1.5) or the proportion of participants with serum transaminases elevation (RR 1.67, 95% CI 0.66 to 4.27; 280 participants; 4 trials; I² = 88%; Analysis 1.6). However, the proportion of participants with total bilirubin elevation was larger in the TACE followed by 3‐DCRT group than in the TACE alone group (RR 2.69, 95% CI 1.34 to 5.40; 172 participants; 2 trials; I² = 0%; Analysis 1.7).
1.5. Analysis.
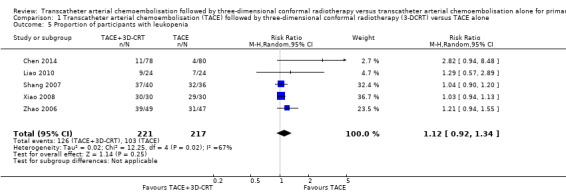
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 5 Proportion of participants with leukopenia.
1.6. Analysis.
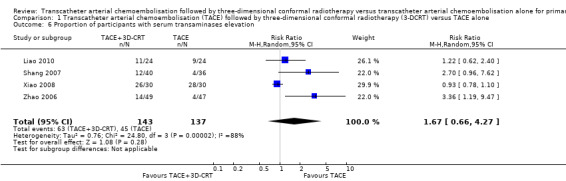
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 6 Proportion of participants with serum transaminases elevation.
1.7. Analysis.
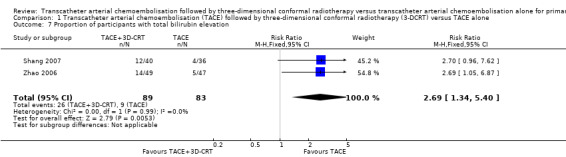
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 7 Proportion of participants with total bilirubin elevation.
Table 2 reports types of adverse events.
1. Non‐serious adverse events.
|
Outcomes/ studies |
TACE followed by 3‐DCTRT | TACE | Both groups | ||||||||||
| 0 | I | II | III | IV | N1 | 0 | I | II | III | IV | N2 | Total | |
| Leukopenia | |||||||||||||
| Chen 2014 | — | — | — | 3 | 11 | — | — | — | 2 | 4 | 15 | ||
| Liao 2010 | — | — | 9 | — | — | 9 | — | — | 7 | — | — | 7 | 16 |
| Shang 2007 | — | 36 | 1 | — | 37 | — | 30 | 2 | — | 32 | 69 | ||
| Xiao 2008 | 0 | 4 | 17 | 8 | 1 | 30 | 1 | 5 | 15 | 7 | 2 | 30 | 60 |
| Zhao 2006 | — | 39 | — | — | 39 | — | 31 | — | — | 31 | 70 | ||
| Serum transaminases elevation | |||||||||||||
| Chen 2014 | — | — | — | — | — | — | — | — | — | — | — | — | 9 |
| Liao 2010 | — | — | 11 | 11 | — | — | 9 | 9 | 20 | ||||
| Ning 2009 | — | — | — | — | — | — | — | — | — | — | — | — | 16 |
| Shang 2007 | — | 10 | 2 | — | 12 | — | 3 | 1 | — | 4 | 16 | ||
| Xiao 2008 | 4 | 12 | 5 | 6 | 3 | 30 | 2 | 17 | 9 | 2 | 0 | 30 | 60 |
| Zhao 2006 | — | 12 | 2 | — | 14 | — | 4 | 0 | — | 4 | 18 | ||
| Nausea and vomiting | |||||||||||||
| Xiao 2008 | 0 | 6 | 6 | 18 | 0 | 30 | 0 | 7 | 7 | 16 | 0 | 30 | 60 |
| Total bilirubin elevation | |||||||||||||
| Shang 2007 | — | — | — | — | — | 12 | — | — | — | — | — | 4 | 16 |
| Zhao 2006 | — | — | — | — | — | 14 | — | — | — | — | — | 5 | 19 |
| Radiation hepatitis | |||||||||||||
| Gong 2011 | — | — | — | — | — | 3 | — | — | — | — | — | — | 3 |
| Liao 2010 | — | — | — | — | — | 1 | — | — | — | — | — | — | 1 |
| Fever | |||||||||||||
| Chen 2014 | — | — | — | — | — | 15 | — | — | — | — | — | 15 | 30 |
| Thrombocytopenia | |||||||||||||
| Shang 2007 | — | 4 | 2 | — | 6 | — | 6 | 3 | — | 9 | 15 | ||
'0 to IV' indicated different degrees of severity for adverse effects; 'N1' indicated the number of participants in the transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) group, 'N2' indicated the TACE alone group, and 'Total' indicated both groups.
Proportion of participants without serum AFP normalisation
We could not perform an analysis of serum AFP as the information reported in the randomised clinical trials was insufficient. Only one trial reported elevation in the level of AFP (Zhao 2006). The rate of participants with serum AFP without decline or normalisation was significantly lower in the TACE followed by 3‐DCRT group than in the TACE group (Chi² = 7.24, P = 0.007).
Subgroup analyses
We could not perform the subgroup analysis of trials at low risk of bias and at high risk of bias because all of the trials were at high risk of bias. Due to data limitations, we could not perform subgroup analyses for the different drugs in TACE; different dosages, frequencies, and ranges in irradiation 3‐DCRT; or aetiology of the chronic liver disease.
Sensitivity analysis
We could not perform sensitivity analysis by excluding studies at high risk of bias. This was due to lack of allocation concealment and blinding of outcome assessment because all trials were at unclear or high risk of bias in these two domains.
We assessed the robustness of our analysis by including only those trials at low risk of bias in incomplete outcome data, and we found that these results did not change the conclusions. There were statistically significant differences for one‐year all‐cause mortality (RR 0.53, 95% CI 0.37 to 0.74; 284 participants; 3 studies; I² = 28%; Analysis 1.8), two‐year all‐cause mortality (RR 0.72, 95% CI 0.58 to 0.88; 284 participants; 3 studies; I² = 0%; Analysis 1.9), three‐year all‐cause mortality (RR 0.84, 95% CI 0.74 to 0.94; 284 participants; 3 studies; I² = 0%; Analysis 1.10), and the rate of participants without tumour response (complete and partial) (RR 0.48, 95% CI 0.33 to 0.68; 284 participants; 3 studies; I² = 0%; Analysis 1.11).
1.8. Analysis.
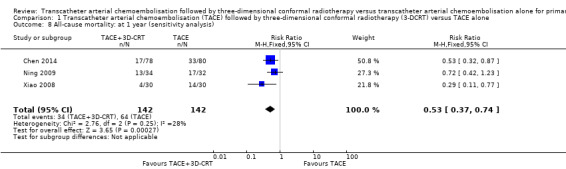
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 8 All‐cause mortality: at 1 year (sensitivity analysis).
1.9. Analysis.
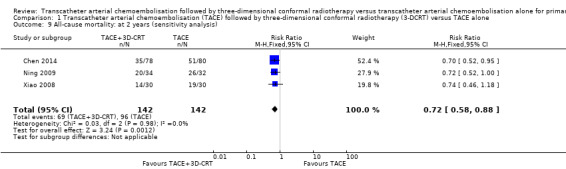
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 9 All‐cause mortality: at 2 years (sensitivity analysis).
1.10. Analysis.
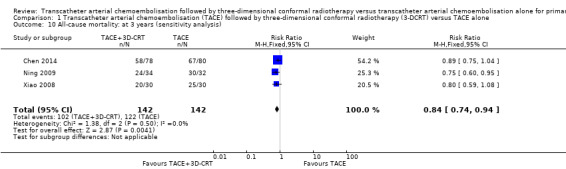
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 10 All‐cause mortality: at 3 years (sensitivity analysis).
1.11. Analysis.
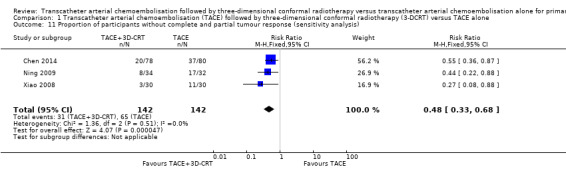
Comparison 1 Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone, Outcome 11 Proportion of participants without complete and partial tumour response (sensitivity analysis).
Risk of random error
In Trial Sequential Analysis (Figure 4; Figure 5; Figure 6; Figure 7), we individually calculated the diversity‐adjusted required information size based upon a proportion of one‐year all‐cause mortality rate of 50%, two‐year all‐cause mortality rate of 70%, three‐year all‐cause mortality rate of 85%, and participants without complete and partial tumour response of 50% in the TACE group; relative risk reductions (RRR) of 20%; an alpha of 2.5% (α) and a beta of 20% (β). All cumulative Z‐curves crossed the monitoring boundary. In summary, the analysis suggested that we have firm evidence to support the effect of TACE followed by 3‐DCRT on the primary outcomes mentioned above. Results obtained by Trial Sequential Analysis indicated that the required information size had been reached for the primary outcomes mentioned above. If the Trial Sequential Analysis was used to assess imprecision, then we would not downgrade the certainty of the evidence by one level for imprecision in GRADE (see below).
4.
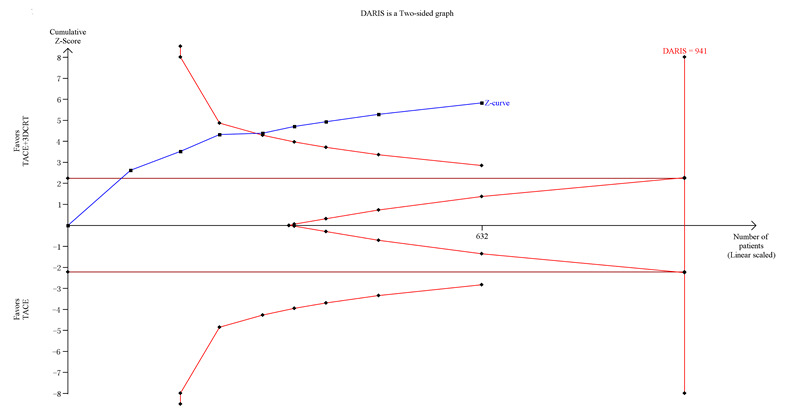
Trial Sequential Analysis of transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE for primary hepatocellular carcinoma with the primary outcome of one‐year all‐cause mortality. The blue line (Z‐curve) shows the cumulative meta‐analysis adding the results of individual trials based on the year of publication. The horizontal green line represents the 2.5% level of significance. The monitoring boundaries (inward sloping red lines) show the significance level after adjusting for the cumulative analysis. The vertical red line shows the required information size (the number of participants needed to determine if firm evidence was established). We conducted the Trial Sequential Analysis with the alpha set to 2.5%, power to 80%, control group event proportion to 50%, relative risk reduction to 20%, and heterogeneity correction based on model variance. The diversity‐adjusted required information size was 941 participants. The cumulative Z‐curve crossed the monitoring boundary before reaching the heterogeneity‐adjusted information size. In total, the cumulative meta‐analysis included 319 participants in the TACE followed by 3‐DCRT group and 313 in the TACE alone group. The cumulative Z‐curve also crossed the monitoring boundary before reaching the adjusted information size when we increased the power to 90%. DARIS: Distributed and Reflective Informatics System.
5.
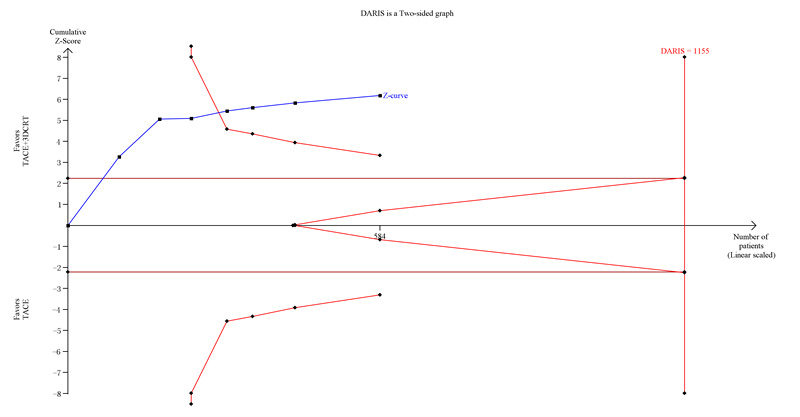
Trial Sequential Analysis of transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone for primary hepatocellular carcinoma with the primary outcome of two‐year all‐cause mortality. The blue line (Z‐curve) shows the cumulative meta‐analysis adding the results of individual trials based on the year of publication. The horizontal line represents the 2.5% level of significance. The monitoring boundaries (inward sloping red lines) show the significance level adjusting for the cumulative analysis. The vertical red line shows the required information size (the number of participants needed to determine if firm evidence was established). We conducted the Trial Sequential Analysis with the alpha set to 2.5%, power to 80%, control group event rate to 70%, relative risk reduction to 20%, and heterogeneity correction based on model variance. The diversity‐adjusted required information size was 1155 participants (diversity adjusted). The cumulative Z‐curve crossed the monitoring boundary before reaching the diversity‐adjusted required information size. In total, the cumulative meta‐analysis included 295 participants in the TACE followed by 3‐DCRT group and 289 in the TACE alone group. The cumulative Z‐curve also crossed the monitoring boundary before reaching the heterogeneity adjusted information size when we increased the power to 90%. DARIS: Distributed and Reflective Informatics System.
6.
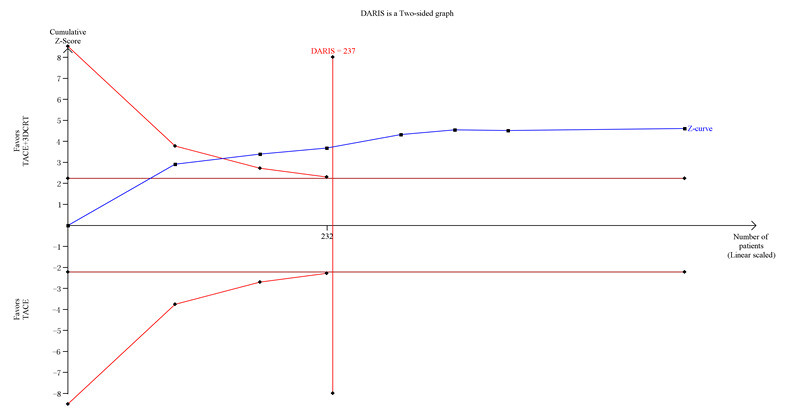
Trial Sequential Analysis of transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT)T versus TACE alone for primary hepatocellular carcinoma with the primary outcome of three‐year all‐cause mortality. The blue line (Z‐curve) shows the cumulative meta‐analysis adding the results of individual trials based on the year of publication. The horizontal green line represents the 2.5% level of significance. The monitoring boundaries (inward sloping red line) show the significance level adjusting for the cumulative analysis. The vertical red line shows the required information size (the number of participants needed to determine if firm evidence was established). We conducted the Trial Sequential Analysis with the alpha set to 5%, power to 80%, control group event rate to 85%, relative risk reduction to 20%, and heterogeneity correction based on model variance. The diversity‐adjusted required information size was 237 participants. The cumulative Z‐curve crossed the monitoring boundary before reaching the heterogeneity‐adjusted information size. In total, the cumulative meta‐analysis included 279 participants in the TACE followed by 3‐DCRT group and 273 in the TACE alone group. The cumulative Z‐curve also crossed the monitoring boundary before reaching the heterogeneity adjusted information size when we increased the power to 90%. DARIS: Distributed and Reflective Informatics System.
7.
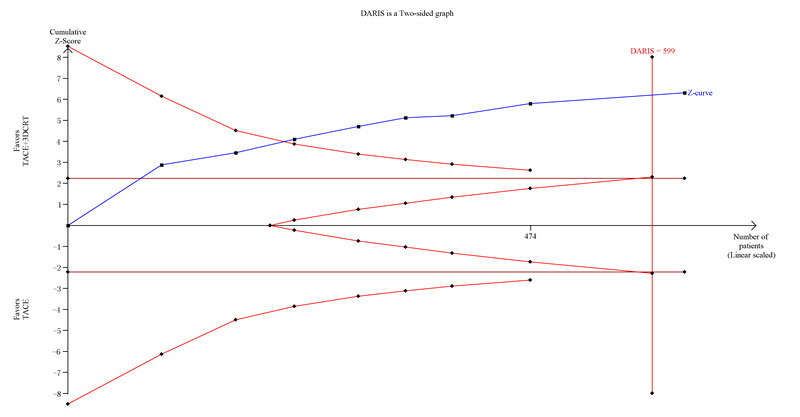
Trial Sequential Analysis of transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone for participants with primary hepatocellular carcinoma without complete or partial tumour response. The blue line (Z‐curve) shows the cumulative meta‐analysis adding the results of individual trials based on the year of publication. The horizontal green line represents the 2.5% level of significance. The monitoring boundary (inward sloping red line) shows the significance level after adjusting for the cumulative analysis. The vertical red line shows the required information size (the number of participants needed to determine if firm evidence was established). We conducted the Trial Sequential Analysis with the alpha set to 2.5%, power to 80%, control group event rate to 50%, relative risk reduction to 25%, and heterogeneity correction based on model variance. The estimated required information size was 599 participants (diversity adjusted). The cumulative Z‐curve crossed the monitoring boundary before reaching the heterogeneity adjusted information size. In total, the cumulative meta‐analysis included 319 participants in the TACE followed by 3‐DCRT group and 313 in the TACE alone group. The cumulative Z‐curve also crossed the monitoring boundary before reaching the heterogeneity adjusted information size when we increased the power to 90%. DARIS: Distributed and Reflective Informatics System.
GRADE assessment
Evidence as evaluated by the GRADE approach was of low certainty for the following outcomes: three‐year all‐cause mortality, and participants without tumour response (complete + partial ). The GRADE evidence was of very‐low certainty for health‐related quality of life; non‐serious adverse events (leukopenia, total bilirubin, and serum transaminases elevation) (Table 1).
Discussion
Summary of main results
This review included eight randomised clinical trials comparing TACE followed by 3‐DCRT versus TACE alone for primary hepatocellular carcinoma, with 632 participants included. Meta‐analyses suggested that TACE followed by 3‐DCRT compared with TACE alone seemed to have a beneficial effect on all‐cause mortality and tumour response (CR+PR), without increasing most of the non‐serious adverse events, but increasing the proportion of participants with elevated total bilirubin. There was no trial heterogeneity in the meta‐analyses of primary outcomes. Trial Sequential Analysis showed that there was low risk of random error. The sensitivity analysis of GRADE and Trial Sequential Analysis assessments found that GRADE downgraded more often for imprecision. Our review findings should be interpreted with caution because of methodological weaknesses in the included trials, resulting in low to very low certainty of evidence.
Overall completeness and applicability of evidence
The trials included in this review compared the efficacy and safety of TACE followed by 3‐DCRT versus TACE alone for people with primary hepatocellular carcinoma. The available randomised clinical trials allowed us to perform meta‐analyses of our primary outcomes. The included trials addressed outcomes such as one‐year all‐cause mortality, two‐year all‐cause mortality, three‐year all‐cause mortality, and participants without complete and partial tumour response. There were no data for serious adverse events. Data were available for the declining rate of AFP in one trial (Zhao 2006), for thrombocytopenia in one trial (Shang 2007), for leukopenia in five trials (Zhao 2006; Shang 2007; Xiao 2008; Liao 2010; Chen 2014), for serum transaminases elevation in four trials (Zhao 2006; Shang 2007; Xiao 2008; Liao 2010), and for total bilirubin elevation in two trials (Zhao 2006; Shang 2007). None of the trials compared different drugs in TACE, different classifications of 3‐DCRT, and the aetiology of chronic liver disease for the primary outcome of all‐cause mortality.
Participants in most of the trials were adults with Child‐Pugh class A/B, with single tumours, and without severe complications or other concerns. Therefore, the data are most applicable to adults who have unresectable primary hepatocellular carcinoma and who are stable and well. In most trials, participants underwent two courses of TACE with a one‐month interval, and 3‐DCRT was delivered one week to four weeks after the last course of TACE, if liver function tests were normal. The sum of the radiation doses in 3‐DCRT therapy ranged from 30 Gy to 66 Gy with 2 Gy/day to 5 Gy/day, 3 days/week to 5 days/week. The most common chemotherapies included 5‐fluorouracil (750 mg to 1250 mg), cisplatin (40 mg to 120 mg), or adriamycin. Embolisation therapy included iodine oil emulsion and gelfoam.
All included trials were performed in inpatient centres in China; hepatocellular carcinoma is one of the most common cancers in China, but it is rare in North America and Europe (Lodato 2006). Although the findings in our review are likely applicable to medical practices in countries with a similar status of primary hepatocellular carcinoma, the question remains of how applicable this evidence is to medical practices in Western countries. In Western countries, chronic hepatitis C and alcoholism are the most common cause of hepatocellular carcinoma. In contrast, chronic hepatitis B is the main cause of hepatocellular carcinoma in China (Kumar 2004; Lodato 2006). Due to the data limitations, we could not perform subgroup analysis based on the aetiology of chronic liver disease. Thus, we were unable to determine the effect of TACE followed by 3‐DCRT in relation to the aetiology of chronic liver disease or different countries.
Quality of the evidence
All included trials were at high risk of bias for selective reporting and blinding (performance bias and detection bias). The GRADE assessment of certainty in the evidence for the analysed outcomes was low to very low because of concerns about the methodological limitations of the included trials (see Table 1). For all outcomes, we downgraded the certainty of the evidence by two levels for risk of bias. Some outcomes were downgraded by one level for imprecision and heterogeneity. A 'low' grade means that further research is likely to have an important impact on our confidence in the estimated effect, and it is likely to change the estimate. A 'very low' grade means that we are uncertain about the estimate. We downgraded the evidence for all outcomes, as most of the included randomised clinical trials had unclear risk of concealment of allocation, non‐blinded assessment of outcomes, attrition bias, or other biases (Figure 2; Figure 3). All biases mentioned above may have affected outcome estimates and confidence. We acknowledge the uncertainty in our results for outcomes mentioned above, and anticipate that future high‐quality trials may change the effect estimates presented in this review.
Potential biases in the review process
We performed a comprehensive literature search to find all relevant studies following the prespecified inclusion criteria of the published protocol (Lu 2016). Two review authors rigorously scanned the reports to avoid selection bias. One issue was reporting bias due to no protocol available for included trials. Thus, the extent of reporting bias could not be assessed, but it might be an issue. The other issue was the method of handling missing data. We considered all participants with entirely missing data as treatment failures and included them in their analysis on ITT basis. However, there are multiple ways to deal with missing data, and there are potential pitfalls with most methods.
Agreements and disagreements with other studies or reviews
We found two previous published meta‐analyses comparing TACE followed by 3‐DCRT with TACE alone for primary hepatocellular carcinoma that included prospective cohort or case‐control studies, but no randomised clinical trials (Zou 2014; Bai 2016). Below are summaries of the results of these two meta‐analyses.
Zou 2014 included 10 prospective cohort or case‐control studies in a meta‐analysis. It observed that TACE followed by 3‐DCRT significantly improved one‐year, two‐year, and three‐year overall survival compared with TACE alone (one‐year odds ratio (OR) 1.87, 95% CI 1.37 to 2.55; two‐year OR 2.38, 95% CI 1.78 to 3.17; three‐year OR 2.97, 95% CI 2.10 to 4.21). In addition, TACE followed by 3‐DCRT was associated with a higher tumour response (OR 3.81, 95% CI 2.70 to 5.37) and declining AFP levels (OR 3.24, 95% CI 2.09 to 5.02). There was no significant heterogeneity or publication bias observed. There were no adverse events reported in the meta‐analysis.
Bai 2016 performed a meta‐analysis of 17 case‐control studies. The results showed that people with hepatocellular carcinoma receiving TACE followed by 3‐DCRT had significantly increased overall survival rates when compared to people receiving TACE alone (one‐year survival rate OR 1.95, 95% CI 1.54 to 2.47; two‐year survival rate OR 1.87, 95% CI 1.49 to 2.34; three‐year survival rate OR 2.00, 95% CI 1.52 to 2.64). There was significant improvement in the tumour response rate in the TACE followed by 3‐DCRT group compared with the TACE alone group (OR 2.29, 95% CI 1.70 to 3.08). There was statistically significant heterogeneity in the two‐year and three‐year survival rates; there was significant publication bias in the one‐year and three‐year survival rates, as well as in tumour response. There were neither AFP nor adverse events reported in this meta‐analysis.
In agreement with the present review, there was a beneficial effect on all‐cause mortality and tumour response assessment for the TACE followed by 3‐DCRT group compared with the TACE alone group. We believe that our review has more reliable results than previously published meta‐analyses, as they included cohort or case‐control studies with more confounding factors and bias affecting the accuracy of result estimates. We only used randomised clinical trials and followed our peer‐reviewed published protocol.
Authors' conclusions
Implications for practice.
Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) may be associated with lower all‐cause mortality and increased tumour response, despite the increased toxicity expressed by a higher rise of total bilirubin. Our review findings should be considered with caution because of the methodological weaknesses in the included trials, resulting in low‐ to very low‐certainty evidence. Data on serious adverse events and health‐related quality of life are lacking. We are also very much uncertain in the results of the reported non‐serious adverse events.
Implications for research.
This review identifies the need for conducting high‐quality randomised clinical trials to evaluate the efficacy of TACE followed by 3‐DCRT versus TACE alone. Randomised clinical trials assessing further the role of TACE followed by 3‐DCRT in people with primary hepatocellular carcinoma are needed. The trials should be performed in people from different countries, with different aetiologies of the chronic liver disease, and the clinical outcomes should be prespecified. In addition, the trials should cover different drugs for TACE, and their dosages, frequency, and range in radiation. Such trials ought to be designed according to the SPIRIT Statements and reported according to the CONSORT Statements. Such trials ought to consider to stratify the participants according to etiology of hepatocellular carcinoma and disease severity. The different classifications of 3‐DCRT should also be studied.
Acknowledgements
We thank all the participants and clinical researchers who were involved in the publications we mentioned in this review. We also give our thanks to the Cochrane Hepato‐Biliary Group for the support they provided.
This work was granted by the First‐class Discipline Construction Foundation of Guangzhou University of Chinese Medicine (Chinese medicine discipline), Young Top Talent Project of Scientific and Technological Innovation in Special Support Plan for Training High‐level Talents in Guangdong (no. 2017TQ04R627) and Guangdong Natural Science Foundation (Project No.2016A030310290).
Peer reviewers: Yuqing Zhang, Canada; Umberto Baccarani, Italy Contact editors: Brian Davidson, UK; Joshua Feinberg, Denmark Sign‐off editor: Christian Gluud, Denmark
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
|
Database |
Period of search | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | May 2018 | (conformal radiotherap* or 3DCRT or 3D‐CRT) AND (((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli* or emboli*)) or TACE or TAE) AND (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | May 2018 | #1 MeSH descriptor: [Radiotherapy, Conformal] explode all trees #2 conformal radiotherap* or 3DCRT or 3D‐CRT #3 #1 or #2 #4 MeSH descriptor: [Embolization, Therapeutic] explode all trees #5 ((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli* or emboli*)) or TACE or TAE #6 #4 or #5 #7 MeSH descriptor: [Carcinoma, Hepatocellular] explode all trees #8 MeSH descriptor: [Liver Neoplasms] explode all trees #9 ((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC #10 #7 or #8 or #9 #11 #3 and #6 and #10 |
| MEDLINE Ovid | 1946 to May 2018 | 1. exp Radiotherapy, Conformal/ 2. (conformal radiotherap* or 3DCRT or 3D‐CRT).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp Embolization, Therapeutic/ 5. (((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli* or emboli*)) or TACE or TAE).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 6. 4 or 5 7. exp Carcinoma, Hepatocellular/ 8. exp Liver Neoplasms/ 9. (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 10. 7 or 8 or 9 11. 3 and 6 and 10 |
| Embase Ovid | 1980 to May 2018 | 1. exp computer assisted radiotherapy/ 2. (conformal radiotherap* or 3DCRT or 3D‐CRT).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp artificial embolism/ 5. (((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli* or emboli*)) or TACE or TAE).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. exp liver cell carcinoma/ 8. exp liver tumor/ 9. (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 10. 7 or 8 or 9 11. 3 and 6 and 10 |
| LILACS (Bireme) | 1982 to May 2018 | (conformal radiotherap$ or 3DCRT or 3D‐CRT) [Words] and (((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli$ or emboli$)) or TACE or TAE) [Words] and (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom$ or cancer$ or neoplasm$ or malign$ or tumo$)) or HCC) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to May 2018 | #4 #3 AND #2 AND #1 #3 TS=(((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #2 TS=(((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli* or emboli*)) or TACE or TAE) #1 TS=(conformal radiotherap* or 3DCRT or 3D‐CRT) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to May 2018 | #4 #3 AND #2 AND #1 #3 TS=(((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #2 TS=(((transarterial or transcatheter or therapeutic or artificial) and (chemoemboli* or emboli*)) or TACE or TAE) #1 TS=(conformal radiotherap* or 3DCRT or 3D‐CRT) |
Data and analyses
Comparison 1. Transcatheter arterial chemoembolisation (TACE) followed by three‐dimensional conformal radiotherapy (3‐DCRT) versus TACE alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality: at 1 year | 8 | 632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.66] |
| 2 All‐cause mortality: at 2 years | 7 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.60, 0.78] |
| 3 All‐cause mortality: at 3 years | 7 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.73, 0.88] |
| 4 Proportion of participants without complete and partial tumour response | 8 | 632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.39, 0.61] |
| 5 Proportion of participants with leukopenia | 5 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.34] |
| 6 Proportion of participants with serum transaminases elevation | 4 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.66, 4.27] |
| 7 Proportion of participants with total bilirubin elevation | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.34, 5.40] |
| 8 All‐cause mortality: at 1 year (sensitivity analysis) | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.37, 0.74] |
| 9 All‐cause mortality: at 2 years (sensitivity analysis) | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.88] |
| 10 All‐cause mortality: at 3 years (sensitivity analysis) | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.94] |
| 11 Proportion of participants without complete and partial tumour response (sensitivity analysis) | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2014.
| Methods | Study design: parallel randomised clinical trial Study duration: March 2000 to March 2009 Duration of follow‐up: until the last follow‐up visit or death from any cause, to December 2012 |
|
| Participants | Country: China Setting: inpatient, 1 centre People with liver function Child‐Pugh class A; ECOG PS 0–1; no cirrhosis, jaundice, or ascites; no severe illnesses except HCC; no history of liver radiotherapy; no contraindications for chemotherapy or radiotherapy; adequate bone marrow, renal, and cardiac function; expected survival > 3 months; aged 18–70 years Number: treatment group 80; control group 78 Mean age (range): treatment group 54 (22–72) years; control group 55 (18–76) years Sex (M/F): treatment group 58/21; control group 60/70 Tumour size: treatment group > 10 cm, 36; ≤ 10 cm, 42; control group > 10 cm, 35; ≤ 10 cm, 45 Tumour lesions (single/multiple): treatment group 52/26; control group 56/24 Stage (III/IV): treatment group 40/39; control group 42/37 |
|
| Interventions | Treatment group: 2 or 3 courses of TACE; 3‐DCRT delivered 2 weeks after the last course of TACE if liver function tests were normal. Radiotherapy was designed with the CMS‐Xi0 radiation treatment planning system. A Varian23EX linear accelerator used to deliver 6 MV high‐energy X‐ray radiotherapy. The sum of the radiation doses received by each participants ranged from 50 Gy to 62 Gy (median 58 Gy) with 2–2.5 Gy/day, 5 days/week. Control group: TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation. 5‐fluorouracil 750–1000 mg; cisplatin 40–60 mg; farmorubicin 40–80 mg; mitomycin 6–10 mg. |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD; CR and PR considered as objective response Serum AFP levels served as complementary information for efficacy evaluation Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: not reported How to deal with adverse reactions (e.g. leukopenia, upper gastrointestinal haemorrhage) not reported No information on compliance and ITT/PP analysis Attempted to contact first author, but no reply has been received yet. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we carried out this randomized controlled trial to compare the efficacy of TACE in combination with 3‐DCRT vs. TACE alone in cases of locally advanced unresectable HCC." Comment: no information of random sequence generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 158 participants included in main analysis of all relevant outcomes |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Low risk | Demographic and baseline characters similar in both groups |
Gong 2011.
| Methods | Study design: parallel randomised clinical trial Study duration: August 2005 to August 2011 Duration of follow‐up: 3 years |
|
| Participants | Country: China Setting: inpatient, 1 centre People with HCC; no obvious bone marrow suppression; no liver or kidney function damage Number: treatment group 24; control group 24 Median age (range): 44 (31–60) years Sex (M/F): 36/12 Median tumour size (range): 7.3 cm (3–16 cm) |
|
| Interventions | Control group: 4 courses of TACE with 1‐month interval. TACE comprised of hepatic arterial infusion chemotherapy and hepatic artery embolisation, cisplatin, fluorouracil, and iodine oil emulsion Treatment group: 2 courses of TACE with 1‐month interval; 3‐DCRT delivered 2 weeks after the last course of TACE, if liver function tests were normal. Radiotherapy: sum of radiation doses received by each participant was 40–60 Gy with 2–5 Gy/dose, 1 dose/day, 3–5 sessions/week |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD; CR and PR considered objective response Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: not reported Attempted to contact first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to a treatment group and to a control group." Comment: random allocation method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Unclear risk | Demographic and baseline characteristics not presented per group. |
Liao 2010.
| Methods | Study design: parallel randomised clinical trial Study duration: November 2005 to November 2007 Duration of follow‐up: date of TACE therapy until the last follow‐up visit (or death) up to November 2009 (median 12 months; range 2–38 months) |
|
| Participants | Country: China Setting: inpatient, 1 centre People with liver function Child‐Pugh class A or B; ECOG PS 0–2; no distant metastasis; no obvious myelosuppression or renal damage Number: treatment group 24; control group 24 Mean age: treatment group 62 years; control group 63 years Sex (M/F): treatment group 15/9; control group 14/10 Tumour size: treatment group 3.5–11 cm; control group 3.8–11.5 cm Child‐Pugh class (A/B): treatment group 17/7; control group 17/7 Stage (III/IV): treatment group 18/6; control group 16/8 |
|
| Interventions | Control group: 3–5 courses of TACE with 1‐month interval. TACE comprised hepatic arterial infusion chemotherapy and hepatic artery embolisation. 5‐fluorouracil 1000–1250 mg; cisplatin 70–90 mg; adriamycin 50–60 mg; peripheral embolisation with iodine oil emulsion and central embolisation with gelfoam Treatment group: 3–5 courses of TACE with 1‐month interval; 3‐DCRT delivered 1–2 weeks after the last course of TACE if liver function tests were normal. Radiotherapy: Varian23EX linear accelerator used to deliver 6 MV high‐energy X‐ray radiotherapy. Sum of radiation doses received by each participants ranged from 40 Gy to 66 Gy (median 55 Gy) with 2–2.5 Gy/day, radiation administered in 20–33 sessions |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD
Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: Project of Quzhou Science and Technology Bureau No information on the compliance and ITT/PP analysis Attempted to contact the first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by drawing lots." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were postrandomisation dropouts and all relevant outcomes were reported for only 45 participants (i.e. 23 in treatment and 22 in control group). |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Low risk | No other potential source of bias identified, baseline characteristics comparable |
Ning 2009.
| Methods | Study design: parallel randomised clinical trial Study duration: March 2002 to March 2005 Duration of follow‐up: not reported |
|
| Participants | Country: China Setting: inpatient, 1 centre People with HCC; Karnofsky score ≥ 70; no cirrhosis, jaundice, or ascites; no severe illnesses except HCC; no history of liver radiotherapy; no contraindications for chemotherapy or radiotherapy; adequate bone marrow, renal, and cardiac function; expected survival > 3 months Number: treatment group 34; control group 32 Mean age (range): 56.5 (37–72) years Sex (M/F): 46/20 |
|
| Interventions | Control group: 4 courses of TACE with 1‐month interval. TACE comprised hepatic arterial infusion chemotherapy and hepatic artery embolisation. 5‐fluorouracil 1000–1250 mg; hydroxyl radical 20 mg; cisplatin 60 mg; mitomycin 14 mg; peripheral embolisation with 10 mL iodine oil emulsion and central embolisation with 1–2 mm gelfoam Treatment group: 2 courses of TACE with 1‐month interval; 3‐DCRT delivered 4 weeks after the last course of TACE if liver function tests were normal. Radiotherapy: a precise linear accelerator used to deliver 6 MV high‐energy X‐ray radiotherapy. The sum of the radiation doses received by each participant ranged from 45 Gy to 55 Gy with 2–5 Gy/day |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD Response rate was the sum of CR+PR AFP
|
|
| Notes | Source of funding: not reported
Baseline characteristics comparison not presented Attempted to contact first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to a treatment group and to a control group." Comment: random allocation method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 66 participants included in main analysis of all relevant outcomes |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Unclear risk | Demographic and baseline characters not presented in either group |
Shang 2007.
| Methods | Study design: parallel randomised clinical trial Study duration: May 2003 to March 2007 Duration of follow‐up: > 6 months |
|
| Participants | Country: China Setting: inpatient, 1 centre People with HCC liver function Child‐Pugh class A or B; tumour size < 6 cm; liver and kidney function normal; no portal vein tumour thrombus; no intratumour dissemination or distant metastasis; no ascites; expected survival > 3 months Number: treatment group 40; control group 36 Median age (range): treatment group 52 (36–68) years; control group 54 (38–70) years Sex (M/F): treatment group 24/16; control group 24/12 Tumour size: treatment group < 3 cm, 26, 3–6 cm, 14; control group < 3 cm, 20; 3–6 cm, 16 Mean AFP: treatment group 834 μg/L; control group 630 μg/L HBV (+/–): treatment group 32/8; control group 30/6 Stage (I/II): treatment group 28/12; control group 22/14 |
|
| Interventions | Control group: 3 courses of TACE with 1‐month interval. TACE comprised hepatic arterial infusion chemotherapy and hepatic artery embolisation. 5‐fluorouracil 1000 mg; cisplatin 40–60 mg; adriamycin 60 mg, or mitomycin C 10–20 mg; peripheral embolisation with iodine oil emulsion 5–20 mL and central embolisation with gelfoam Treatment group: 2 courses of TACE with 1‐month interval; 3‐DCRT delivered 3 weeks after the last course of TACE if liver function tests were normal. Radiotherapy: the sum of the radiation doses received by each participant was ≤ 30 Gy with 2 Gy/day, 1 session/day, 5 days/week, total course: 4–6 weeks |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: not reported No information on the compliance and ITT/PP analysis Attempted to contact the first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to a treatment group and to a control group." Comment: random allocation method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on whether the analysis included lost data |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Low risk | Demographic and baseline characters similar in both groups |
Xiao 2008.
| Methods | Study design: parallel randomised clinical trial Study duration: January 2002 to June 2006 Duration of follow‐up: > 6 months |
|
| Participants | Country: China Setting: inpatient, 1 centre People with I–IIIa stage of primary liver cancer without celiac lymph nodes or distant metastasis; with liver function Child‐Pugh class A or B; Karnofsky score ≥ 70; WBC ≥ 3.0 × 10⁹/L, PLT ≥ 5.0 × 10¹²/L; imaging examination can confirm pathological lesions and surgical resection could not be performed Number: treatment group 30; control group 30 Mean age (range): treatment group (16–75 years); control group (17–78 years) Sex (M/F): treatment group 22/8; control group 23/7 Tumour size: treatment group 2.8–14.5 cm; control group 2.5–16 cm Child‐Pugh class (A/B): treatment group 19/11; control group 20/10 Stage (I/II/III): treatment group 10/12/8; control group 12/13/5 |
|
| Interventions | Control group: 2 courses of TACE with 1‐month interval. TACE comprised hepatic arterial infusion chemotherapy and hepatic artery embolisation. 5‐fluorouracil 1000–1250 mg; cisplatin 100 mg; adriamycin 50–100 mg; peripheral embolisation with iodine oil emulsion 10–30 mL and central embolisation with gelfoam 1–2 mm. Treatment group: 2 courses of TACE with 1‐month interval; 3‐DCRT delivered 1–3 weeks after the last course of TACE if liver function tests were normal. Radiotherapy: Varian23EX linear accelerator used to deliver 6 MV high‐energy X‐ray radiotherapy. The sum of the radiation doses received by each participant was 55 Gy, 5 Gy/session, 5 sessions/week |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD
Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: not reported How to deal with several adverse reactions (e.g. fever, abdominal pain) not mentioned No information on compliance and ITT/PP analysis Attempted to contact the first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to a treatment group and to a control group by random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 60 participants included in main analysis of all relevant outcomes |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Low risk | Demographic and baseline characters similar in both groups |
Xiao 2011.
| Methods | Study design: parallel randomised clinical trial Study duration: January 2008 to December 2010 Duration of follow‐up (mean): 2 years |
|
| Participants | Country: China Setting: inpatient, 1 centre People with primary liver cancer; normal blood examinations; renal and cardiac function; no portal vein tumour thrombus; no intratumour dissemination or distant metastasis; no ascites; expected survival > 3 months Number: treatment group 40; control group 40 Median age (range): treatment group 40 (20–72) years; control group 39 (25–63) years Sex (M/F): treatment group 29/11; control group 28/12 Tumour size: treatment group ≤ 5 cm, 25; > 5 cm, 15; control group ≤ 5 cm, 23; > 5 cm, 17 Mean AFP: treatment group ≤ 400 μg/L, 22; > 400 μg/L, 18; control group ≤ 400 μg/L, 25; > 400 μg/L, 15 Tumour number (single/multiple): treatment group 31/9; control group 29/11 Stage (III/IV): treatment group 34/6; control group 35/5 |
|
| Interventions | Control group: 1 or 2 courses of TACE with 1‐month interval. TACE comprised hepatic arterial infusion chemotherapy and hepatic artery embolisation. Cisplatin 60–120 mg; adriamycin 30–100 mg; peripheral embolisation with iodine oil emulsion 10–30 mL, and central embolisation with gelfoam Treatment group: TACE with 3‐DCRT. 3‐DCRT delivered by 6 MV high‐energy X‐ray radiotherapy. The sum of the radiation doses received by each participant ranged from 50 Gy to 60 Gy, 2 Gy/day, 5 days/week |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: not reported Cases of "lost to follow‐up" reported, but no information on ITT/PP analysis mentioned Attempted to contact the first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on whether the analysis included lost data |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Low risk | Demographic and baseline characters similar in both groups |
Zhao 2006.
| Methods | Study design: parallel randomised clinical trial Study duration: January 1998 to April 2000 Duration of follow‐up: > 6 months |
|
| Participants | Country: China Setting: inpatient, 1 centre People with primary liver cancer; Karnofsky score ≥ 70; normal liver function; tumour size < 6 cm; no portal vein tumour thrombus; no intratumour dissemination or distant metastasis; no ascites Number: treatment group 49; control group 47 Median age (range): treatment group 53 (32–70) years; control group 52 (36–69) years Sex (M/F): treatment group 32/17; control group 28/19 Tumour size: treatment group < 3 cm, 28; 3–6 cm, 21; control group < 3 cm, 25; 3–6 cm, 22 AFP ≥ 400 ng/mL: treatment group 42; control group 42 Stage (I/II): treatment group 36/13; control group 31/16 |
|
| Interventions | Control group: 4 courses of TACE with 1‐month interval. TACE comprised hepatic arterial infusion chemotherapy and hepatic artery embolisation. 5‐fluorouracil 750 mg; hydroxyl radical 15 mg; cisplatin 40 mg Treatment group: 2 courses of TACE; 3‐DCRT delivered 3 weeks after the last course of TACE. Radiotherapy: Varian23EX linear accelerator used to deliver 3‐DCRT. The sum of the radiation doses received by each participant was 45–55 Gy, 4–5 Gy/session, every other day |
|
| Outcomes | According to RECIST, treatment efficacy divided into CR, PR, SD, and PD; CR and PR considered as objective response Response rate was the sum of CR+PR
|
|
| Notes | Source of funding: not reported Cases of "lost to follow‐up" reported, but no information on ITT/PP analysis mentioned Attempted to contact the first author, without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to a treatment group and to a control group." Comment: random allocation method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on the distribution of lost data or whether the analysis included the lost data |
| Selective reporting (reporting bias) | High risk | 1 predefined outcome (serious adverse events) not reported. Protocol not available |
| Other bias | Low risk | Demographic and baseline characters similar in both groups |
3‐DCRT: three‐dimensional conformal radiotherapy; AFP: alpha fetoprotein; CR: complete response; ECOG PS: Eastern Cooperative Oncology Group performance status; F: female; HCC: hepatocellular carcinoma; ITT: intention to treat; M: male; NCI‐CTC: National Cancer Institute Common Toxicity Criteria (now known as Common Terminology Criteria for Adverse Events (CTCAE)); PD: progressive disease; PLT: platelets; PP: per protocol; PR: partial response; RECIST: response evaluation criteria of solid tumours; SD: stable disease; TACE: transcatheter arterial chemoembolisation; WBC: white blood cell count.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chia‐Hsien 2001 | Not a randomised clinical trial and the control group was not 3‐DCRT. |
| Chung 2006 | Not a randomised clinical trial |
| Guo 2005 | Not a randomised clinical trial, but a retrospective study |
| Koo 2010 | Not a randomised clinical trial |
| Liang 2005 | Not a randomised clinical trial, but a retrospective study |
| Lu 2015 | Not a randomised clinical trial, but a retrospective study |
| Shim 2005 | Not a randomised clinical trial, but a retrospective study |
| You 2007 | Not a randomised clinical trial, but a retrospective study |
| Zeng 2004 | Not a randomised clinical trial, but a retrospective study |
| Zhang 2009 | Not a randomised clinical trial, but a retrospective study |
3‐DCRT: three‐dimensional conformal radiotherapy.
Differences between protocol and review
Change in review title: during the review process, we observed that in most trials, participants underwent two courses of TACE or more with a one‐month interval, and 3‐DCRT was delivered one week to four weeks after the last course of TACE when liver function tests were normal. So, for greater accuracy, we changed the original title "Combination of three‐dimensional conformal radiotherapy and transcatheter arterial chemoembolisation versus transcatheter arterial chemoembolisation for primary hepatocellular carcinoma" into "Transcatheter arterial chemoembolisation followed by three‐dimensional conformal radiotherapy versus transcatheter arterial chemoembolisation alone for primary hepatocellular carcinoma in adults."
We added two new authors (CT and NX) at the review stage as they provided overall guidance and supervision, and contributed to the revision of the review.
We changed the order of the secondary outcomes as follows: "health‐related quality of life; non‐serious adverse events; and proportion of participants without serum AFP normalisation". Previously, the order of the outcomes was: "proportion of participants without serum AFP normalisation; health‐related quality of life; and non‐serious adverse events". We changed the order of the outcomes as health‐related quality of life and non‐serious adverse events are more important to people with hepatocellular carcinoma than the outcome 'proportion of participants without serum AFP normalisation'.
Contributions of authors
LL: designed, codeveloped, and drafted the protocol and review; identified studies, extracted data, assessed risk of bias. ZJ: designed, codeveloped, and drafted the protocol and review; identified studies, extracted data, assessed risk of bias. WZ: provided overall guidance and supervision, and contributed to the revision of the review; resolved disagreements regarding identified studies. CT: provided overall guidance and supervision, and contributed to the revision of the review. NX: provided overall guidance and supervision, and contributed to the revision of the review.
All review authors approved the final version of this review for publication.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
LL: none ZJ: none WZ: none CT: none NX: none
These authors contributed equally to this work.
These authors contributed equally to this work.
New
References
References to studies included in this review
Chen 2014 {published data only}
- Chen WJ, Yuan SF, Zhu LJ, Sun XN, Zheng W. Three‐dimensional conformal radiotherapy in combination with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma. Journal of B.U.ON. 2014;19(3):692‐7. [PubMed] [Google Scholar]
Gong 2011 {published data only}
- Gong QY, Li JG, Kang GL. Effects of transcatheter arterial chemoembolization (TACE) versus TACE combined with three dimensional radiation (3‐DCRT) in the treatment of patients with primary hepatocellular carcinoma. Practical Journal of Cancer 2011;26(6):634‐6. [Google Scholar]
Liao 2010 {published data only}
- Liao XF, He HJ, Zhou ZS, Hu W, Zhu XP. Three dimensional conformal radiotherapy combined with interventional therapy for primary hepatocellular carcinoma. Journal of Practical Oncology 2010;25(6):681‐4. [Google Scholar]
Ning 2009 {published data only}
- Ning SH, Li GF, Huang YS, Li GS. Three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Sichuan Medical Journal 2009;30(12):1896‐8. [Google Scholar]
Shang 2007 {published data only}
- Shang Y, You GX, Xu HY, Chen MC. Prospective randomised clinical study of transcatheter arterial chemoembolization, combined with three‐dimensional conformal radiotherapy for primary liver cancer: an analysis of 40 cases. World Chinese Journal of Digestology 2007;15(29):3140‐2. [Google Scholar]
Xiao 2008 {published data only}
- Xiao ZM, Ouyang TC, Yu RZ, Jiang XS, Ren H, Wu ZJ. Transcatheter arterial chemoembolization combined with 3‐dimensional conformal radiotherapy for patients with unresectable primary hepatic carcinoma. Chinese Journal of Clinical Oncology 2008;35(1):18‐21. [Google Scholar]
Xiao 2011 {published data only}
- Xiao P, Dong Y, Sun Y, Liu QF, Cao HM, Xu ZY, et al. Effect comparison of TACE plus 3DCRT and TACE alone in the treatment of primary hepatic cancer. Journal of Basic and Clinical Oncology 2011;24(6):506‐7. [Google Scholar]
Zhao 2006 {published data only}
- Zhao MH, Lang FP, Jiang QA, Ma JJ, Song YX. Three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for inoperable primary liver cancer. Chinese Journal of Radiation Oncology 2006;15(1):39‐41. [Google Scholar]
References to studies excluded from this review
Chia‐Hsien 2001 {published data only}
- Chia‐Hsien Cheng J, Chuang VP, Cheng SH, Lin YM, Cheng TI, Yang PS, et al. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. International Journal of Cancer 2001;96(4):243‐52. [DOI] [PubMed] [Google Scholar]
Chung 2006 {published data only}
- Chung YL, Jian JJ, Cheng SH, Tsai SY, Chuang VP, Soong T, et al. Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clinical Cancer Research 2006;12(9):2706‐15. [DOI] [PubMed] [Google Scholar]
Guo 2005 {published data only}
- Guo JX, Sun XN, Huang MX, Chen J, Xu ZY, Ma QB. Evaluation of the efficacy for alternated treatment on primary liver cancer by interventional therapy in combination with fractionated stereotactic conformal radiotherapy. Chinese Journal of Clinical Oncology 2005;32(24):1418‐20. [Google Scholar]
Koo 2010 {published data only}
- Koo JE, Kim JH, Lim YS, Park SJ, Won HJ, Sung KB, et al. Combination of transarterial chemoembolization and three‐dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. International Journal of Radiation Oncology, Biology, Physics 2010;78(1):180‐7. [DOI] [PubMed] [Google Scholar]
Liang 2005 {published data only}
- Liang SX, Zhu XD, Lu HJ, Pan CY, Li FX, Huang QF, et al. Hypofractionated three‐dimensional conformal radiation therapy for primary liver carcinoma. Cancer 2005;103(10):2181‐8. [DOI] [PubMed] [Google Scholar]
Lu 2015 {published data only}
- Lu DH, Fei ZL, Zhou JP, Hu ZT, Hao WS. A comparison between three‐dimensional conformal radiotherapy combined with interventional treatment and interventional treatment alone for hepatocellular carcinoma with portal vein tumour thrombosis. Journal of Medical Imaging and Radiation Oncology 2015;59(1):109‐14. [DOI] [PubMed] [Google Scholar]
Shim 2005 {published data only}
- Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver International 2005;25(6):1189‐96. [DOI] [PubMed] [Google Scholar]
You 2007 {published data only}
- You CR, Jang JW, Kang SH, Bae SH, Choi JY, Yoon SK, et al. Efficacy of transarterial chemolipiodolization with or without 3‐dimensional conformal radiotherapy for huge HCC with portal vein tumor thrombosis. Korean Journal of Hepatology 2007;13(3):378‐86. [DOI] [PubMed] [Google Scholar]
Zeng 2004 {published data only}
- Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer Journal (Sudbury, Mass.) 2004;10(5):307‐16. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3‐dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer 2009;115(6):1245‐52. [DOI] [PubMed] [Google Scholar]
Additional references
Bai 2016
- Bai H, Gao P, Gao H, Sun G, Dong C, Han J, et al. Improvement of survival rate for patients with hepatocellular carcinoma using transarterial chemoembolization in combination with three‐dimensional conformal radiation therapy: a meta‐analysis. Medical Science Monitor 2016;22:1773‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Bronowicki 1994
- Bronowicki JP, Vetter D, Dumas F, Boudjema K, Bader R, Weiss AM, et al. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4‐year study of 127 French patients. Cancer 1994;74(1):16‐24. [DOI] [PubMed] [Google Scholar]
Cabrera 2010
- Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Alimentary Pharmacology & Therapeutics 2010;31(4):461‐76. [DOI] [PubMed] [Google Scholar]
Cammà 2002
- Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta‐analysis of randomized controlled trials. Radiology 2002;224(1):47‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochranesystematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(110):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cha 2005
- Cha C, Dematteo RP. Molecular mechanisms in hepatocellular carcinoma development. Best Practice & Research. Clinical Gastroenterology 2005;19(1):25‐37. [DOI] [PubMed] [Google Scholar]
Cheng 2000
- Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. International Journal of Radiation Oncology, Biology, Physics 2000;47(2):435‐42. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deuffic 1998
- Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet 1998;351(9097):214‐5. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Serag 2001
- El‐Serag HB. Epidemiology of hepatocellular carcinoma. Clinics in Liver Disease 2001;5(1):87‐107, vi. [DOI] [PubMed] [Google Scholar]
El‐Serag 2008
- El‐Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134(6):1752‐63. [DOI] [PubMed] [Google Scholar]
El‐Serag 2014
- El‐Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go?. Hepatology (Baltimore, Md.) 2014;60(5):1767‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2011
- Feng M, Ben‐Josef E. Radiation therapy for hepatocellular carcinoma. Seminars in Radiation Oncology 2011;21(4):271‐7. [DOI] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. Journal International du Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Grade Working Group 2004‐2007, 2008.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence – study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence – publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence – inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence – indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables – binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles – continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Guyatt 2013d
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso‐Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14‐22. [PUBMED: 28529188] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Ikeda 1991
- Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer 1991;68(10):2150‐4. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(120):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kothary 2007
- Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. Journal of Vascular and Interventional Radiology : JVIR 2007;18(12):1517‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumar 2004
- Kumar V, Fausto N, Abbas A. Robbins & Cotran Pathologic Basis of Disease. 7th Edition. Philadelphia (PA): Saunders, 2004. [Google Scholar]
Lan 1983
- Lan GK, DeMets GL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659‐63. [Google Scholar]
Lawrence 1990
- Lawrence TS, Tesser RJ, Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. International Journal of Radiation Oncology, Biology, Physics 1990;19(4):1041‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee JH, Wu HG, Kim HJ, Park CI, Lee SH, Kim DW, et al. Hypofractionated three‐dimensional conformal radiotherapy for medically inoperable early stage non‐small‐cell lung cancer. Radiation Oncology Journal 2013;31(1):18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li SH, Guo ZX, Xiao CZ, Wei W, Shi M, Chen ZY, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pacific Journal of Cancer Prevention 2013;14(8):4759‐63. [DOI] [PubMed] [Google Scholar]
Liang 2015
- Liang HY, Guo QY, Sun W, Mao XN, Wen F, Shan M, et al. Sequential use of transhepatic arterial chemoembolization and bipolar radiofrequency ablation in the clinical therapy of hepatocellular carcinoma. Cancer Biotherapy and Radiopharmaceuticals 2015;30(10):427‐32. [DOI] [PubMed] [Google Scholar]
Llovet 2002
- Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359(9319):1734‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Llovet 2003
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology (Baltimore, Md.) 2003;37(2):429‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Llovet 2004
- Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplantation 2004;10:S115‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lo 2002
- Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2002;35(5):1164‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lodato 2006
- Lodato F, Mazzella G, Festi D, Azzaroli F, Colecchia A, Roda E. Hepatocellular carcinoma prevention: a worldwide emergence between the opulence of developed countries and the economic constraints of developing nations. World Journal of Gastroenterology 2006;12(45):7239‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsuura 1998
- Matsuura M, Nakajima N, Arai K, Ito K. The usefulness of radiation therapy for hepatocellular carcinoma. Hepato‐Gastroenterology 1998;45(21):791‐6. [MEDLINE: ] [PubMed] [Google Scholar]
McGlynn 2001
- McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF Jr. International trends and patterns of primary liver cancer. International Journal of Cancer. Journal International du Cancer 2001;94(2):290‐6. [DOI] [PubMed] [Google Scholar]
Mittal 2013
- Mittal S, El‐Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. Journal of Clinical Gastroenterology 2013;47 Suppl:S2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Murata 2014
- Murata S, Mine T, Sugihara F, Yasui D, Yamaguchi H, Ueda T, et al. Interventional treatment for unresectable hepatocellular carcinoma. World Journal of Gastroenterology 2014;20(37):13453‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Nakamura 1990
- Nakamura H, Hashimoto T, Oi H, Sawada S, Furui S, Mizumoto S, et al. Treatment of hepatocellular carcinoma by segmental hepatic artery injection of adriamycin‐in‐oil emulsion with overflow to segmental portal veins. Acta Radiologica 1990;31(4):347‐9. [PubMed] [Google Scholar]
Oliveri 2011
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD004787.pub2] [DOI] [PubMed] [Google Scholar]
Park 2014
- Park ES, Kwon do H, Park JB, Lee do H, Cho YH, Kim JH, et al. Gamma Knife surgery for treating brain metastases arising from hepatocellular carcinomas. Journal of Neurosurgery 2014;121 Suppl:102‐9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 1993
- Robertson JM, Lawrence TS, Dworzanin LM, Andrews JC, Walker S, Kessler ML, et al. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. Journal of Clinical Oncology 1993;11(7):1286‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasaki 1987
- Sasaki Y, Imaoka S, Kasugai H, Fujita M, Kawamoto S, Ishiguro S, et al. A new approach to chemoembolization therapy for hepatoma using ethiodized oil, cisplatin, and gelatin sponge. Cancer 1987;60(6):1194‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane Reviews: the ROBES Meta‐Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Seong 2000
- Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. International Journal of Radiation Oncology, Biology, Physics 2000;47(5):1331‐5. [DOI] [PubMed] [Google Scholar]
Spieth 2003
- Spieth K, Kaufmann R, Gille J. Metronomic oral low‐dose treosulfan chemotherapy combined with cyclooxygenase‐2 inhibitor in pretreated advanced melanoma: a pilot study. Cancer Chemotherapy and Pharmacology 2003;52(5):377‐82. [DOI] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non‐randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takayasu 1987
- Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, et al. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology 1987;163(2):345‐51. [DOI] [PubMed] [Google Scholar]
Takayasu 2006
- Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131(2):461‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 27 May 2017).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA – Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Venook 2010
- Venook AP, Papandreou C, Furuse J, Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15 Suppl 4:5‐13. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. Cancer. Fact sheet N°297, 2009. www.who.int/mediacentre/factsheets/fs297/en/ (accessed 30 June 2015).
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamagami 2014
- Yamagami T, Yoshimatsu R, Ishikawa M, Kajiwara K, Aikata H, Tashiro H, et al. Transcatheter arterial chemoembolization with an interventional‐CT system for recurrent hepatocellular carcinoma after living donor liver transplantation. Hepato‐gastroenterology 2014;61(133):1387‐92. [PubMed] [Google Scholar]
Yoshikawa 1994
- Yoshikawa M, Saisho H, Ebara M, Iijima T, Iwama S, Endo F, et al. A randomized trial of intrahepatic arterial infusion of 4'‐epidoxorubicin with Lipiodol versus 4'‐epidoxorubicin alone in the treatment of hepatocellular carcinoma. Cancer Chemotherapy and Pharmacology 1994;33 Suppl:S149‐52. [DOI] [PubMed] [Google Scholar]
Yu 2014
- Yu JI, Park HC. Considerations for radiation therapy in hepatocellular carcinoma: the radiation oncologists' perspective. Digestive Diseases (Basel, Switzerland) 2014;32(6):755‐63. [DOI] [PubMed] [Google Scholar]
Zhou 2007
- Zhou ZH, Liu LM, Chen WW, Men ZQ, Lin JH, Chen Z, et al. Combined therapy of transcatheter arterial chemoembolisation and three‐dimensional conformal radiotherapy for hepatocellular carcinoma. British Journal of Radiology 2007;80(951):194‐201. [DOI] [PubMed] [Google Scholar]
Zou 2014
- Zou LQ, Zhang BL, Chang Q, Zhu FP, Li YY, Wei YQ, et al. 3D conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World Journal of Gastroenterology 2014;20(45):17227‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lu 2016
- Lu L, Zeng J, Wen Z. Combination of three‐dimensional conformal radiotherapy and transcatheter arterial chemoembolisation versus transcatheter arterial chemoembolisation for primary hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012244] [DOI] [PMC free article] [PubMed] [Google Scholar]


