Abstract
The mammalian lipin 1 phosphatidate phosphatase is a key regulatory enzyme in lipid metabolism. By catalyzing phosphatidate dephosphorylation, which produces diacylglycerol, the enzyme plays a major role in the synthesis of triacylglycerol and membrane phospholipids. The importance of lipin 1 to lipid metabolism is exemplified by cellular defects and lipid-based diseases associated with its loss or overexpression. Phosphorylation of lipin 1 governs whether it is associated with the cytoplasm apart from its substrate or with the endoplasmic reticulum membrane where its enzyme reaction occurs. Lipin 1β is phosphorylated on multiple sites, but less than 10% of them are ascribed to a specific protein kinase. Here, we demonstrate that lipin 1β is a bona fide substrate for casein kinase II (CKII), a protein kinase that is essential to viability and cell cycle progression. Phosphoamino acid analysis and phosphopeptide mapping revealed that lipin 1β is phosphorylated by CKII on multiple serine and threonine residues, with the former being major sites. Mutational analysis of lipin 1β and its peptides indicated that Ser-285 and Ser-287 are both phosphorylated by CKII. Substitutions of Ser-285 and Ser-287 with nonphosphorylatable alanine attenuated the interaction of lipin 1β with 14-3-3β protein, a regulatory hub that facilitates the cytoplasmic localization of phosphorylated lipin 1. These findings advance our understanding of how phosphorylation of lipin 1β phosphatidate phosphatase regulates its interaction with 14-3-3β protein and intracellular localization and uncover a mechanism by which CKII regulates cellular physiology.
Keywords: phosphatidate, diacylglycerol, triacylglycerol, 14–3-3 protein, protein kinase, protein phosphorylation, phosphatase, casein kinase II, cell signaling, lipid droplet, lipin 1 PA phosphatase, membrane phospholipid
Introduction
PA3 phosphatase plays a central role in lipid metabolism (1–9) (Fig. 1). By catalyzing the dephosphorylation of PA to produce diacylglycerol (10), the enzyme has a major impact on the synthesis of triacylglycerol and membrane phospholipids and on the abundance of the signaling molecule PA (1–9) (Fig. 1). Three genes encode PA phosphatase in humans (i.e. LPIN1, LPIN2, and LPIN3) and mice (i.e. Lpin1, Lpin2, and Lpin3) (11–13). Their protein products (i.e. lipin 1 (α, β, and γ isoforms), 2, and 3) share conserved domains at the N-terminal (NLIP) and C-terminal (CLIP) regions (11) (Fig. 2). The PA phosphatase activity depends on a conserved glycine within NLIP and the DXDX(T/V) catalytic motif in the haloacid dehalogenase–like domain within CLIP (14–16) (Fig. 2). Lipin 1 also has a transcriptional co-activator function that depends on the LXXIL motif in CLIP (17) (Fig. 2).
Figure 1.
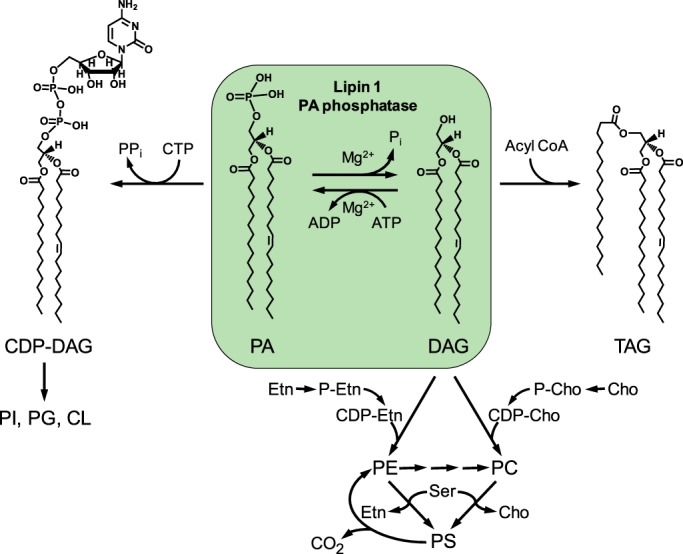
Central role of PA phosphatase in the synthesis of triacylglycerol and membrane phospholipids in mammalian cells. The structures of CDP-diacylglycerol, PA, diacylglycerol, and triacylglycerol are shown with the C16:0 and C18:1 fatty acyl groups. PA phosphatase plays a major role in governing whether cells utilize PA for the synthesis of triacylglycerol via diacylglycerol or for the synthesis of membrane phospholipids via CDP-diacylglycerol. The PA phosphatase reaction is counterbalanced by the conversion of diacylglycerol to PA. The major phospholipids phosphatidylcholine and phosphatidylethanolamine are synthesized from the PA-derived diacylglycerol via the CDP-choline and CDP-ethanolamine branches, respectively, of the Kennedy pathway. Phosphatidylcholine is also synthesized from phosphatidylethanolamine by the three-step methylation reactions using AdoMet as a methyl donor. Phosphatidylserine is derived from phosphatidylcholine or phosphatidylethanolamine via a base-exchange reaction, and its decarboxylation produces phosphatidylethanolamine. In addition to their roles in lipid synthesis, PA and diacylglycerol are known to facilitate membrane fission/fusion events (96–101) and play roles in vesicular trafficking (102–106). More comprehensive pathways of lipid synthesis may be found in Ref. 2. DAG, diacylglycerol; TAG, triacylglycerol; PI, phosphatidylinositol; PG, phosphatidylglycerol; CL, cardiolipin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; Cho, choline; Etn, ethanolamine.
Figure 2.

Domains/regions and phosphorylation sites of mouse lipin 1β. The diagram shows the conserved NLIP (blue) and CLIP (haloacid dehalogenase (HAD)-like) (green) domains found at the N and C terminus, respectively; the conserved G in NLIP and the catalytic (DXDXT) and transcriptional co-activator (LXXIL) motif sequences in CLIP; the nuclear localization signal/polybasic sequence (NLS/PBS, purple) region; and the serine-rich region (S, red). The serine (S) and threonine (T) residues known to be phosphorylated (42, 47–51) are grouped at their approximate regions in the protein, and those examined in this study are colored in red. The sites phosphorylated by CKI, CKII (this study), and mTORC1 are indicated.
The importance of PA phosphatase to lipid metabolism is exemplified in humans and mice by cellular defects and lipid-based diseases associated with loss or overexpression of the enzyme activity. The loss of lipin 1 PA phosphatase activity causes rhabdomyolysis in humans and mice (18, 19), and the deficiency in mice is also characterized by hepatic steatosis during the neonatal period, lipodystrophy, insulin resistance, and peripheral neuropathy (11, 20). Polymorphisms in the human LPIN1 gene are associated with insulin resistance and the metabolic syndrome (21), whereas the overexpression of the Lpin1 gene in mice results in increased lipogenesis and obesity (22). Other disease conditions linked to lipin 1 PA phosphatase activity include inflammation and atherosclerosis (23, 24), liver disease (25–29), and cancer (30–34). Loss of human lipin 2 PA phosphatase activity causes chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anemia (Majeed syndrome) (35, 36), whereas genetic variations in the LPIN2 gene are linked to type 2 diabetes (37). So far, no defects have been ascribed to the loss of lipin 3 PA phosphatase activity.
The mode of action and control of PA phosphatase activity is primarily regulated by its cellular location (3, 4, 38). Mouse lipin 1 is found in the cytoplasm, endoplasmic reticulum, and nucleus (4, 39, 40), and its cellular location is regulated by covalent modifications that include phosphorylation (41–43), sumoylation (44), acetylation (45), and interaction with 14-3-3 proteins (46). The phosphorylated form of lipin 1 is enriched in the cytoplasm, where it is physiologically inactive, whereas its dephosphorylated form is enriched in the membrane, where it catalyzes the PA phosphatase reaction (42, 43). The membrane association of the enzyme is facilitated by acetylation (45), whereas its association with the cytoplasm is facilitated by interaction with 14-3-3 proteins (46). The localization of lipin 1α to the nucleus, where it functions as a transcriptional coactivator (17), is facilitated by its sumoylation (44).
Phosphoproteomics and targeted phosphorylation studies have shown that mouse lipin 1β is phosphorylated on multiple serine and threonine residues (42, 47–51) (Fig. 2). Except for mTORC1, which phosphorylates Ser-106 and Ser-472 (42, 47), and CKI, which phosphorylates Ser-483 and Ser-487 (52), the identities of the protein kinases that phosphorylate the remaining sites are unknown. Identifying protein kinases for lipin 1β phosphorylation is important to provide information on the signaling networks involved in its regulation under normal and disease states (53). In this work, we demonstrate that lipin 1β is phosphorylated by CKII, a conserved serine/threonine protein kinase that is essential for viability and cell cycle progression in mammalian cells (54–56). We show that CKII phosphorylates lipin 1β at Ser-285 and Ser-287. Moreover, the CKII phosphorylation of lipin 1β at Ser-285 and Ser-287 facilitates its interaction with 14-3-3β protein. This work advances the understanding of the phosphorylation-mediated regulation of lipin 1β PA phosphatase and demonstrates an unrecognized mechanism by which CKII regulates cell physiology.
Results
Lipin 1β is a substrate for CKII
Lipin 1β PA phosphatase is the predominant isoform in most tissues, and it is known to be phosphorylated on multiple residues (42, 47). The bioinformatics analysis of lipin 1β indicates that it is phosphorylated by a plethora of protein kinases (57). Of the putative kinases, CKII has a high probability of phosphorylating lipin 1β (57). Given this prediction, we examined whether lipin 1β is a target substrate for CKII. For this analysis, we used a purified preparation of mouse FLAG-tagged lipin 1β that was expressed in HeLa cells (Fig. 3) and examined its phosphorylation by following the incorporation of the radioactive phosphate from [γ-32P]ATP into the protein. The analysis of the reaction products by SDS-PAGE and phosphorimaging showed that lipin 1β is phosphorylated by CKII (Fig. 4A). By phosphoamino acid analysis, the 32P-labeled lipin 1β was shown to contain phosphate on the residues of serine and threonine, with the former being a major site (76% of the total phosphorylation) (Fig. 4B). When lipin 1β was phosphorylated by CKII after pretreatment with λ-phosphatase (58) to remove phosphates from its endogenous phosphorylation, it did not show a difference in the extent of phosphorylation. This result indicates that the in vitro phosphorylation of lipin 1β is not affected by its endogenous phosphorylation. We further characterized the lipin 1β phosphorylation to confirm that it is a bona fide substrate for CKII. The protein kinase activity depended on the amount of CKII (Fig. 5A), the time of the reaction (Fig. 5B), the amount of lipin 1β (Fig. 5C), and the concentration of ATP (Fig. 5D).
Figure 3.
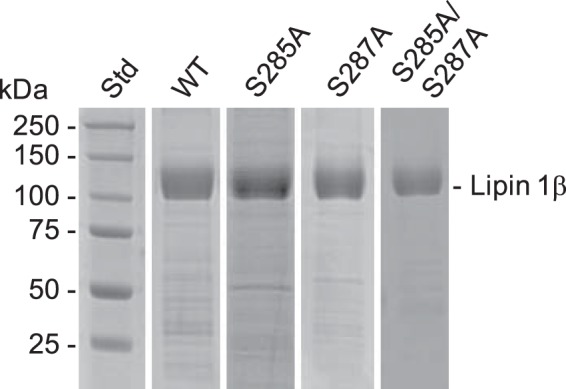
SDS-PAGE analysis of purified mouse lipin 1β proteins. Mouse FLAG-tagged lipin 1β was expressed in HeLa cells and purified by affinity chromatography. Samples of the purified preparations of the WT (3.9 μg), S285A (1.7 μg), S287A (4.8 μg), and S285A/S287A (1.4 μg) lipin 1β were subjected to SDS-PAGE and stained with Coomassie Blue. The positions of lipin 1β and molecular mass standards (Std) are indicated.
Figure 4.
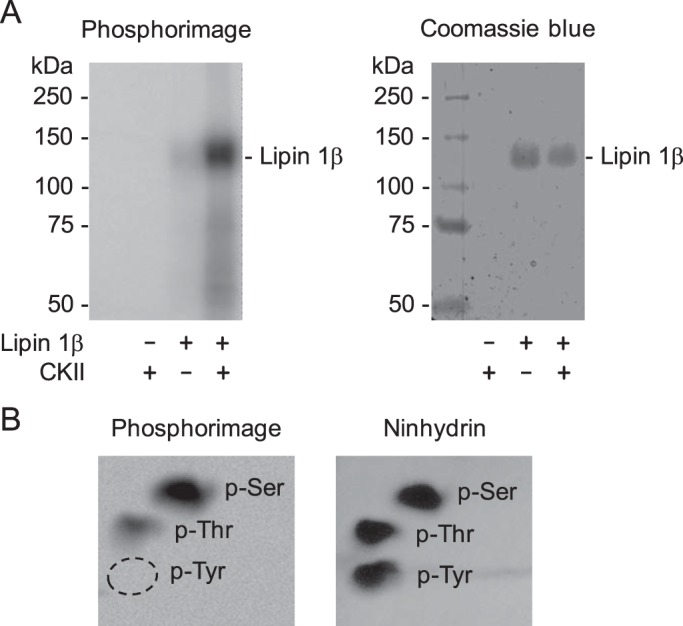
Lipin 1β is phosphorylated by CKII on the serine and threonine residues. A, purified WT FLAG-tagged lipin 1β (0.3 μg) was incubated for 15 min at 30 °C in the presence (+) or absence (−) of 0.2 μg of CKII and [γ-32P]ATP (2,000 cpm/pmol). The reaction mixtures were resolved by SDS-PAGE and subjected to phosphorimaging (left), followed by staining with Coomassie Blue (right). The positions of lipin 1β and the molecular mass standards are indicated. B, 32P-labeled WT lipin 1β was incubated with 6 n HCl for 90 min at 110 °C. The acid hydrolysate was mixed with standard phosphoamino acids and resolved by two-dimensional electrophoresis on a cellulose TLC plate, which was subjected to phosphorimaging (left) and staining with ninhydrin (right). The positions of phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated. The experiments in A and B were repeated three times, and the data shown are representative.
Figure 5.

CKII activity on lipin 1β depends on the reaction time and the amounts of CKII, lipin 1β, and ATP. Lipin 1β was incubated with CKII and [γ-32P]ATP at 30 °C, and its phosphorylation was monitored by following the incorporation of the radioactive phosphate into the protein. The reaction mixtures were spotted onto nitrocellulose papers, which were washed with 75 mm phosphoric acid and subjected to scintillation counting. The CKII reaction was conducted by varying the amount of CKII (A), the reaction time (B), the amount of lipin 1β (C), and the concentration of ATP (D). A, B, and C, 50 μm ATP; A, C, and D, 15 min; A, B, and D, 0.1 μg of lipin 1β; B, C, and D, 0.2 μg of CKII. The data shown are means ± S.D. (error bars) from triplicate assays.
To determine whether the catalytic function of lipin 1β is affected by its phosphorylation, the CKII-treated enzyme was measured for PA phosphatase activity by following the release of Pi from PA using the Triton X-100/PA-mixed micellar assay. Compared with the untreated control, which had a specific activity of 6 μmol/min/mg, the CKII-phosphorylated lipin 1β showed no significant difference in PA phosphatase activity.
CKII phosphorylates lipin 1β in the serine-rich region
The phosphorylation of the serine-rich region of lipin 1α facilitates its interaction with 14-3-3 proteins to promote a cytoplasmic localization (46). However, the protein kinase(s) involved in the phosphorylation is unknown. Analysis of the serine-rich region of lipin 1β, which is identical to that of lipin 1α, with the Phosphomotif Finder (59) and NetPhos (57) programs indicates that seven residues (e.g. Ser-281, Thr-282, Ser-285, Ser-287, Ser-291, Ser-293, and Thr-298) are putative sites for phosphorylation by CKII. To examine whether the residues are the phosphorylation sites of CKII, we utilized a 22-residue synthetic peptide whose sequence (RPSTPKSDSELVSKSADRLTPK) was derived from the serine-rich region of lipin 1β (residues 279–300). The phosphorylation of the lipin 1β peptide was monitored by following the incorporation of the radioactive phosphate from [γ-32P]ATP into the peptide. The CKII activity on the lipin 1β peptide depended on the amount of protein kinase (Fig. 6A) and the time of the reaction (Fig. 6B). The CKII activity was also examined with respect to the concentrations of the WT peptide (Fig. 6C) and ATP (Fig. 6D). In both cases, CKII activity followed Michaelis–Menten kinetics with apparent Vmax and Km values, respectively, for the WT peptide of 45 ± 5 nmol/min/mg and 146 ± 40 μm and for ATP of 10.7 ± 0.8 nmol/min/mg and 6.2 ± 1.5 μm. These results indicated that the lipin 1β peptide (i.e. serine-rich region) is phosphorylated by CKII.
Figure 6.
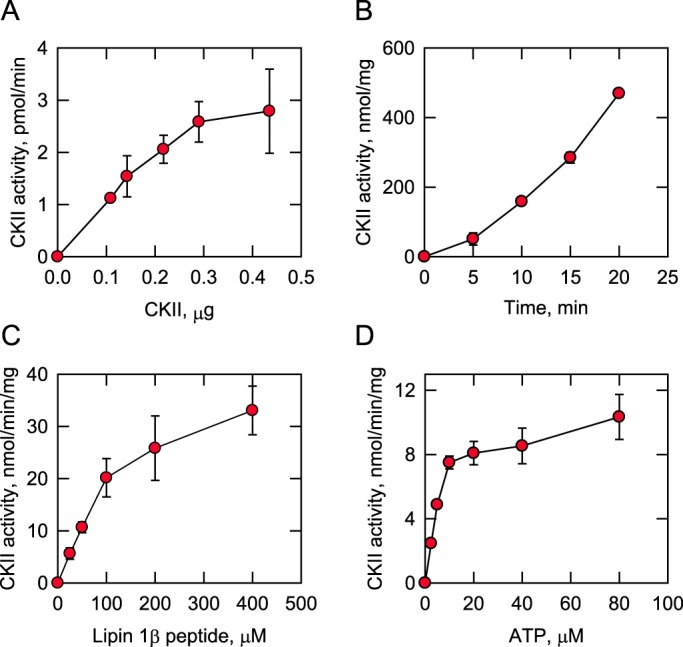
CKII activity on the lipin 1β peptide depends on the reaction time and the amount of CKII, the lipin 1β peptide, and ATP. The lipin 1β peptide (RPSTPKSDSELVSKSADRLTPK) was incubated with CKII and [γ-32P]ATP at 30 °C, and its phosphorylation was monitored by following the incorporation of the radioactive phosphate into the peptide. The reaction mixtures were spotted onto P81 phosphocellulose papers, which were washed with 75 mm phosphoric acid and subjected to scintillation counting. The CKII reaction was conducted by varying the amount of CKII (A), the reaction time (B), the concentration of the lipin 1β peptide (C), and the concentration of ATP (D). A, B, and C, 100 μm ATP; A, C, and D, 15 min; A, B, and D, 100 μm lipin 1β peptide; B, C, and D, 0.2 μg of CKII. The data shown are means ± S.D. (error bars) from triplicate assays.
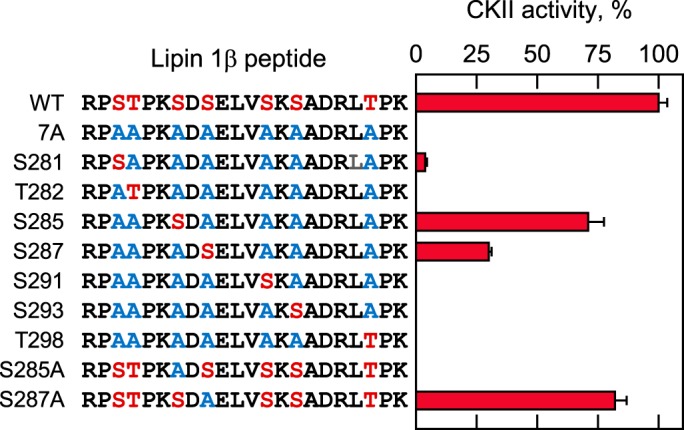
Mutational analysis of the lipin 1β peptide identifies Ser-285 and Ser-287 as sites of phosphorylation by CKII
After determining the phosphorylation of the serine-rich region by CKII, we examined which of the seven serine/threonine residues is a target phosphorylation site. For this purpose, CKII activity was measured on the derivatives of the lipin 1β peptide in which the alanine residue was substituted for the Ser or Thr residue (Fig. 7). A mutant peptide (referred to as 7A) in which all putative phosphorylation sites were changed to the alanine residues (blue color) served as a negative control for CKII activity. In seven peptides, one putative phosphorylation site was left intact (red color), and the remaining six putative sites were changed to the alanine residues (blue color). Of these peptides, the S285 and S287 peptides were phosphorylated by CKII at 71 ± 6.5 and 30 ± 1.2%, respectively, of the WT peptide phosphorylation (Fig. 7). In contrast, the other mutant peptides were phosphorylated at the level of 4% or less (Fig. 7). These results indicate that Ser-285 and Ser-287 of the serine-rich region are the phosphorylation sites of CKII.
Figure 7.
Analysis of lipin 1β peptides by CKII identifies Ser-285 and Ser-287 as target phosphorylation sites. The indicated WT and mutant peptides (100 μm) were incubated with 0.2 μg of CKII and 100 μm [γ-32P]ATP for 15 min at 30 °C, and the peptide phosphorylation was measured by following the incorporation of the radioactive phosphate into the peptide. The reaction mixtures were spotted onto P81 phosphocellulose papers, which were washed with 75 mm phosphoric acid and then subjected to scintillation counting. The phosphorylation levels of mutant peptides were normalized to that of the WT peptide. The data shown are means ± S.D. (error bars) from triplicate assays.
The phosphorylation of Ser-285 and Ser-287 was further examined by mutating the serine residues individually to alanine (i.e. S285A and S287A, blue color) without altering the remaining putative phosphorylation sites (red color) (Fig. 7). No CKII activity was observed on the S285A peptide, whereas the activity on the S287A peptide was 82 ± 4.8% of that observed for the WT peptide. The phosphorylation of the S287A mutant peptide could be attributed to the phosphorylation of Ser-285, but it is unclear why no phosphorylation was observed for the S285A peptide because Ser-287 is available for phosphorylation.
The effects of the S285A, S287A, and S285A/S287A mutations on the phosphorylation of lipin 1β by CKII
To confirm that Ser-285 and Ser-287 of lipin 1β are the sites of phosphorylation by CKII, the serine residues, alone and in combination, were changed to alanine. The mutant lipin 1β proteins purified after their expression in HeLa cells (Fig. 3) were treated with CKII and [γ-32P]ATP and then analyzed by SDS-PAGE and phosphorimaging. The S285A and S287A mutations did not show a major effect on the overall extent of lipin 1β phosphorylation, suggesting that it is also phosphorylated on many other sites. To address this possibility, the CKII-phosphorylated WT lipin 1β was digested with TPCK-treated trypsin, and the resulting peptides were separated by electrophoresis and TLC. This analysis showed multiple phosphopeptides from the CKII-phosphorylated lipin 1β, indicating that the protein is phosphorylated on many sites (Fig. 8). The analysis of lipin 1β with the PeptideCutter program (60) predicts that Ser-285 and Ser-287 should be contained in the same peptide. Consistent with this prediction, the phosphopeptide map of the S285A/S287A double mutant showed the loss (indicated by the dashed circle) of a single phosphopeptide (indicated by the white arrow in the phosphopeptide map of WT lipin 1β) (Fig. 8). Moreover, this phosphopeptide was present (indicated by the white arrow) in the phosphopeptide map of the S287A mutant protein, which is consistent with the phosphorylation of Ser-285 (Fig. 8). However, the same phosphopeptide was missing (indicated by the dashed circle) in the phosphopeptide map of the S285A mutant (Fig. 8). This result suggested that the phosphorylation of Ser-287 was affected by the loss of phosphorylation on Ser-285.
Figure 8.
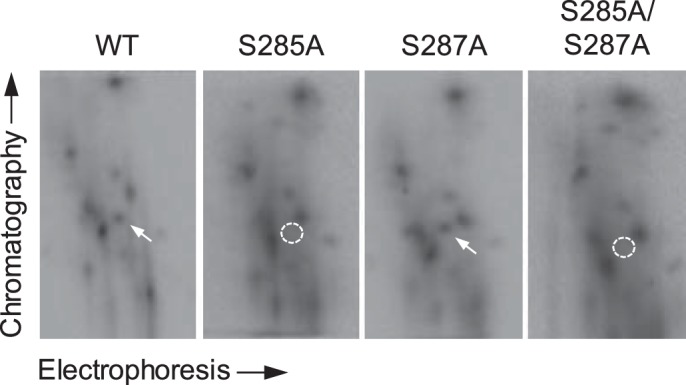
Phosphopeptide mapping analysis of WT, S285A, S287A, and S285A/S287A forms of lipin 1β. The WT, S285A, or S285A/S287A form of lipin 1β (0.1 μg) was phosphorylated by 0.2 μg of CKII with 50 μm [γ-32P]ATP. The phosphorylated proteins were resolved by SDS-PAGE and transferred to a PVDF membrane. The 32P-labeled lipin 1β proteins on the membrane were digested with TPCK-treated trypsin. The phosphopeptides produced by the proteolytic digestion were separated on cellulose TLC plates by electrophoresis (from left to right) in the first dimension and by chromatography (from bottom to top) in the second dimension. The white arrow indicates a phosphopeptide that is absent (dashed circle) in the maps of the S285A and S285A/S287A mutants. The data shown are representative of two independent experiments.
The effects of the S285A, S287A, and S285A/S287A mutations on the interaction of lipin 1β with 14-3-3β protein
We examined the interaction of GST-tagged 14-3-3β protein with the WT and S285A, S287A, and S285A/S287A forms of FLAG-tagged lipin 1β. Consistent with the previous finding on the interaction of lipin 1α with 14-3-3β (46), lipin 1β was shown to interact with GST-14-3-3β protein (Fig. 9A). That the protein–protein interaction was specific is indicated by the inability of GST alone to interact with the lipin 1β protein (Fig. 9B). The S285A, S287A, and S285A/S287A mutations caused reductions in the interaction of lipin 1β with the 14-3-3β protein by 47, 67, and 43%, respectively, when compared with the WT control (Fig. 9D). Whereas these reductions caused by the individual and combined mutations were significant, the mutational effects were not significantly different from each other (Fig. 9D).
Figure 9.
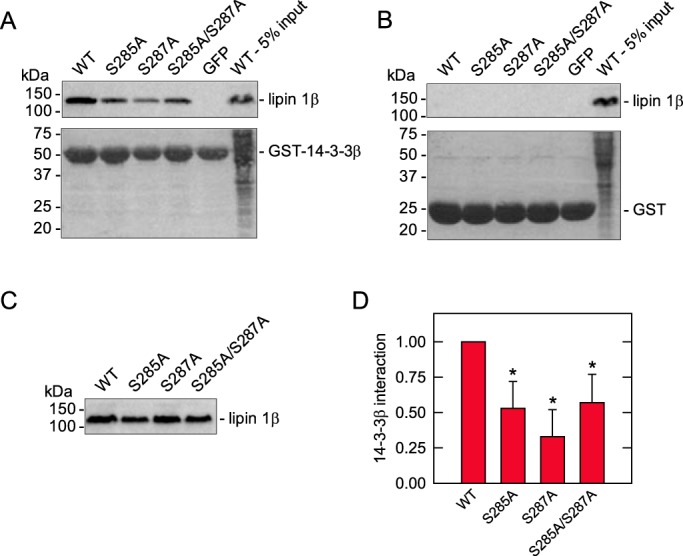
Effects of the phosphorylation-deficient mutations of lipin 1β on its interaction with 14-3-3β protein. GSH-Sepharose-bound GST-14-3-3β (A) or GST (B) was incubated with the HEK-293T cell lysate (1 mg of protein) containing the WT or the S285A, S287A, of S285A/S287A mutant form of lipin 1β at 4 °C for 2 h. Following the collection of the resin and extensive washing, the proteins were eluted and separated by SDS-PAGE. The resolved proteins were transferred to PVDF membrane and analyzed for lipin 1β by immunoblotting with anti-FLAG antibodies. The lipin 1β signals on the immunoblots were acquired with a Fuji LAS 4000 analyzer and quantified with Multi Gauge software. The GST-14-3-3β and GST proteins on the blots were visualized by staining with Ponceau S. A blot showing the lipin 1β input (5%) of the experiment is shown in C, and the quantification of the data is shown in D. The lipin 1β signal that interacted with the GST-14-3-3β protein was divided by the signal of the lipin 1β input; the value obtained for each mutant protein was normalized to the WT control value that is arbitrarily set at 1.0. GFP was included as a negative control. The positions of lipin 1β, GST-14-3-3, GST, and molecular mass standard are indicated. The data shown in A–C are representative of four independent experiments, whereas the data in D are the average of the four experiments ± S.D. (error bars). *, p < 0.05 versus WT.
Discussion
PA phosphatase has emerged as a key regulatory enzyme in eukaryotic lipid metabolism (8, 22, 61, 62). This is exemplified by the plethora of physiological defects and disease states imparted by the loss of its function to catalyze the conversion of PA to DAG (8, 22, 61, 62). The posttranslational modification of phosphorylation largely governs whether the enzyme is associated with the cytoplasm apart from its substrate PA or with the membrane where the PA phosphatase reaction occurs (8, 22, 61, 62). Lipin 1β PA phosphatase contains many serine and threonine residues that are phosphorylated (42, 47–51), but less than 10% of the sites can be ascribed to a specific protein kinase and signaling network (41, 42, 47, 52). In this work, a bioinformatics approach was taken to identify protein kinases responsible for the phosphorylation of lipin 1β. Phosphoamino acid analysis showed that lipin 1β is phosphorylated by CKII on the serine and threonine residues, and the enzymological analysis of CKII activity indicated that lipin 1β is a bona fide substrate for the kinase.
The phosphopeptide mapping experiment of the CKII-phosphorylated lipin 1β indicated that it is phosphorylated on multiple sites. To manageably identify the sites of phosphorylation, we focused on the serine-rich region of the protein. Péterfy et al. (46) have previously shown that the phosphorylation of the serine-rich region of lipin 1α is required for its interaction with 14-3-3 proteins and retention in the cytoplasm. Because the phosphorylations of Ser-252, Ser-254, and Ser-260 within the serine-rich region are critical for the cytoplasmic localization of lipin 1α (46) and the serine residues are putative CKII phosphorylation sites, we hypothesized that the corresponding residues, namely Ser-285, Ser-287, and Ser-293, in lipin 1β are phosphorylated by CKII. The enzymological analysis of the WT and mutant versions of the lipin 1β peptide corresponding to the serine-rich region led to the conclusion that Ser-285 and Ser-287 are CKII phosphorylation sites, with Ser-285 being the major site. Ser-293 was not identified as a CKII phosphorylation site by the assay employed in this study. On one hand, the phosphorylation assays with the lipin 1β mutant peptide substrates (e.g. S285A and S287A) indicated that lack of phosphorylation on Ser-285 prevented the phosphorylation of Ser-287, whereas the lack of phosphorylation on Ser-287 did not affect the phosphorylation of Ser-285. On the other hand, CKII phosphorylated the lipin 1β peptide S287, and in this peptide, Ser-285 is changed to an alanine. Yet the phosphopeptide-mapping experiments with the full-length lipin 1β S285A and S287A mutants also indicated that the phosphorylation of Ser-285 affects the phosphorylation of Ser-287. The reason for this puzzlement is unclear.
Like lipin 1α (46), the lipin 1β associated with 14-3-3β protein, and this association was attenuated by the CKII phosphorylation-deficient S285A, S287A, and S285A/S287A mutations. The phosphorylation of lipin 1β by CKII did not affect its PA phosphatase activity in vitro. Thus, the effect of the CKII-mediated phosphorylation of lipin 1β would be expected to inhibit PA phosphatase function by sequestration of the enzyme in the cytoplasm. The interaction of lipin 1β with 14-3-3β protein is expected to be more complex than just phosphorylation of a couple of sites (46), and thus, more work is needed to identify the protein kinase(s) that phosphorylate other residues within the serine-rich region of the protein. Additional work is also required to identify the CKII target sites outside the serine-rich region and clarify their role in regulating lipin 1β function.
To date, 44 serine/threonine residues have been identified as sites of phosphorylation in lipin 1β (42, 47–51) (Fig. 2). Of the identified sites, mTORC1 phosphorylates Ser-106 and Ser-472 (42, 47), and CKI phosphorylates Ser-483 and Ser-487 (52). Insulin stimulation is followed by the phosphorylation of lipin 1β by mTORC1 (41, 42, 47), whereas the phosphorylation by CKI causes lipin 1β to interact with the SCFβ-TRCP E3 ubiquitin ligase complex for its ubiquitination and degradation (52). These phosphorylations are hierarchical in nature; the phosphorylation by CKI requires prephosphorylation by mTORC1 (52).
The yeast Pah1 PA phosphatase also plays an important role in lipid metabolism and cell physiology (8, 63), and like lipin 1β, the enzyme localization (i.e. cytoplasmic versus membrane) is governed by its phosphorylation (3, 64). Pah1 is subject to multiple (i.e. 34) phosphorylations (64–75), and some of the protein kinases involved have been identified and the sites mapped (76–80). These include protein kinases A (76) and C (77), the cyclin-dependent protein kinases Cdc28/CDK1 (78) and Pho85/CDK5 (79), and CKII (80). Phosphorylations by the cyclin-dependent protein kinases and protein kinase A sequester Pah1 in the cytoplasm (76, 78, 79, 81). The phosphorylations by Pho85/CDK5 and protein kinase A (76) also reduce the PA phosphatase activity of Pah1 (76, 79). The phosphorylation of Pah1 by CKII has little effect on PA phosphatase activity, but it inhibits the subsequent phosphorylation by protein kinase A (80). The phosphorylation by protein kinase C does not affect the location or catalytic activity of Pah1, but it facilitates the proteolytic degradation of the enzyme by the 20S proteasome (77, 82).
In this work, we focused on CKII because the kinase is essential to viability and cell cycle progression from yeast to humans (54–56, 83, 84), and it has been identified as one of the protein kinases that regulates yeast Pah1 PA phosphatase (80). In the context of lipin 1β and signaling in mammalian cells, CKII is activated by insulin (85–87). One of the insulin-mediated targets of CKII is acetyl-CoA carboxylase (87), the enzyme that catalyzes the committed step in the synthesis of fatty acids (2). Fatty acids are the building blocks of triacylglycerol and membrane phospholipids, lipid molecules whose synthesis is also controlled by the activity of PA phosphatase (1–9). Like lipin 1β, the phosphorylation of acetyl-CoA carboxylase by many protein kinases results in the attenuation of its function (88). Overall, the work reported here not only advances the understanding of lipin 1β phosphorylation, it also sheds new light on the CKII-mediated regulation of lipid metabolism.
Experimental procedures
Materials
All chemicals were reagent grade or better. Dioleoyl PA was obtained from Avanti Polar Lipids. Protein assay reagents, electrophoresis reagents, protein molecular mass standards, and Coomassie Blue R-250 were from Bio-Rad. Leupeptin and pepstatin were purchased from Cayman Chemical. Cellulose TLC plates were from EMD Millipore. InstantBlue protein stain was purchased from Expedeon. Peptides were prepared by EZBiolab. GSH-Sepharose, pGEX4T3, pGEX4T3-14-3-3β, PVDF membrane, and the enhanced chemifluorescence Western blotting reagent were purchased from GE Healthcare Life Sciences. CKII (α2β2) was purchased from New England Biolabs (catalogue no. P6010S). PerkinElmer Life Sciences and National Diagnostics, respectively, were the sources of radiochemicals and scintillation counting supplies. Sigma-Aldrich was the source of BSA, nitrocellulose, Ponceau S, phosphoamino acid standards, PMSF, mouse anti-FLAG (DYKDDDDK) M2 antibody (catalogue no. F3165), alkaline-conjugated goat anti-mouse antibody (catalogue no. A3562), and TPCK-treated trypsin. P81 phosphocellulose paper was from Whatman.
Preparation of lipin 1β proteins
The S285A, S287A, and S285A/S287A mutations in mouse lipin 1β were made using PCR site-directed mutagenesis in the pcDNA3 vector with FLAG-tagged lipin 1β inserts. The mutagenesis was confirmed by DNA sequencing. The FLAG-tagged lipin 1β cDNAs were placed into pAdTRACK-CMV; the shuttle vector was recombined with pAdEasy, and adenovirus was made by transformation of the linearized recombined plasmid in HEK-293 cells. The FLAG-tagged lipin 1β proteins were expressed in HeLa cells by adenoviral infection and purified by affinity chromatography as described by Granade and Harris (58). Analysis of the proteins by SDS-PAGE indicated that they were purified to ∼90% of homogeneity. The amounts of lipin 1β proteins resolved in the SDS-polyacrylamide gel were quantified using BSA as a standard (58).
For the GST-14-3-3β interaction studies, the FLAG-tagged WT or mutant forms of lipin 1β were expressed in HEK-293T by transient transfection. HEK-293T cells in a 10-cm plate were transfected with 5 μg of pcDNA3-FLAG-Lpin1β (WT, S285A, S287A, or S285A/S287A). Three days post-transfection, the cells were scraped in 10 mm Na2HPO4 buffer (pH 7.4) containing 50 mm β-glycerophosphate, 50 mm NaF, 1 mm EDTA, 1 mm EGTA, 0.1% Nonidet P-40, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 1 mm PMSF and centrifuged at 16,000 × g for 10 min at 4 °C to obtain the clarified cell lysate.
Phosphorylation of lipin 1β by CKII
CKII activity on lipin 1β was measured by following the incorporation of the radioactive phosphate of [γ-32P]ATP into the substrate. The assays were performed in triplicate for 15 min at 30 °C in a total volume of 20 μl. The reaction mixture contained 25 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 10 mm β-mercaptoethanol, 0.2 μg of CKII, 50 μm [γ-32P]ATP, and 0.1 μg of lipin 1β or 100 μm lipin 1β peptide unless otherwise indicated. The kinase reaction was terminated by the addition of 5× Laemmli sample buffer (89) and subjected to SDS-PAGE to separate 32P-labeled lipin 1β from [γ-32P]ATP, and protein was transferred to a PVDF membrane unless otherwise indicated. Radioactively labeled lipin 1β was visualized by phosphorimaging, and the extent of the phosphorylation was quantified by ImageQuant software. Alternatively, the reaction was terminated by spotting the mixture onto nitrocellulose paper, which was then washed three times with 75 mm phosphoric acid to remove unreacted radioactive ATP. The nitrocellulose paper was then subjected to scintillation counting. For the phosphorylation of lipin 1β peptides, the reaction was terminated by spotting the reaction mixture onto a P81 phosphocellulose paper, followed by phosphoric acid washing and scintillation counting. One unit of CKII activity was defined as 1 nmol/min, and specific activity was defined as the units per mg of CKII.
Analysis of phosphopeptides and phosphoamino acids
The 32P-labeled lipin 1β transferred to PVDF membrane was digested with TPCK-treated trypsin for phosphopeptide mapping and hydrolyzed with 6 n HCl at 110 °C for phosphoamino acid analysis (90–92). The tryptic digests were separated on the cellulose plates first by electrophoresis and then by TLC (90–92). The acid hydrolysates were mixed with standard phosphoamino acids and separated by two-dimensional electrophoresis on the cellulose plates. Radioactive phosphopeptides and phosphoamino acids were visualized by phosphorimaging analysis. Nonradioactive phosphoamino acid standards were visualized by ninhydrin staining.
Preparation of GST-14-3-3β protein
Escherichia coli BL21 (DE3) pLysS cells were transformed with pGEX4T3 or pGEX4T3-14-3-3β. The E. coli transformant was inoculated into 800 ml of lysogeny broth medium containing 100 μg/ml ampicillin and grown to A600 nm = 0.5. Transgene expression was induced with the addition of 0.5 mm isopropyl-β-d-1-thiogalactopyranoside, and cultures were incubated for an additional 2 h. The E. coli culture was harvested by centrifugation and lysed by sonication in PBS (pH 7.4) containing 0.5% Nonidet P-40, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 1 mm PMSF. The sonicate was centrifuged at 16,000 × g for 10 min at 4 °C, and the supernatant was used as cell lysate. The lysate containing the overexpressed GST or GST-14-3-3β (850 μg of protein) was incubated with GSH-Sepharose at 4 °C for 1 h, followed by washing of the resin three times with sonication buffer.
Analysis of lipin 1β interaction with GST-14-3-3β protein
GSH-Sepharose–bound GST-14-3-3β or GST was incubated with the HEK-293T lysate (1 mg of protein) containing the WT or the S285A, S287A, and S285A/S287A mutant forms of lipin 1β at 4 °C for 2 h with rotation and washed three times with 10 mm Na2HPO4 buffer (pH 7.4) containing 50 mm β-glycerophosphate, 50 mm NaF, 1 mm EDTA, 1 mm EGTA, 0.1% Nonidet P-40, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 1 mm PMSF, followed by two additional washes with the same buffer containing 450 mm NaCl. Proteins were eluted from GSH-Sepharose by incubation in Laemmli's sample buffer (89) at 70 °C, followed by SDS-PAGE, transfer to PVDF membrane, and immunoblot analysis (93). Mouse anti-FLAG and alkaline-conjugated goat anti-mouse antibodies were used at dilutions of 1:1,000 and 1:30,000, respectively (94). GST-14-3-3β or GST on the PVDF membrane was visualized by staining with Ponceau S. The images of immunoblot signals and Ponceau S staining were acquired with a Fuji LAS 4000 analyzer, and lipin 1β proteins were quantified with Multi Gauge software.
PA phosphatase assay
PA phosphatase activity was measured by following the release of water-soluble Pi from chloroform-soluble PA using the Triton X-100/PA-mixed micellar assay as described by Han and Carman (13). The reaction mixture contained 160 mm Tris-HCl (pH 7.5) buffer, 1 mm MgCl2, 10 mm 2-mercaptoethanol, 0.2 mm dioleoyl PA, 2 mm Triton X-100, and lipin 1β protein in a total volume of 10 μl. Water-soluble Pi was measured with the malachite green–molybdate reagent at A650 nm (13, 95). Enzyme assays were conducted in triplicate, and the average S.D. value of the assays was ±5%.
Analyses of data
SigmaPlot software was used to determine kinetic parameters according to the Michaelis–Menten equation. The statistical analyses were performed with SigmaPlot or GraphPad Prism software.
Author contributions
M. H., M. E. G., G.-S. H., T. E. H., and G. M. C. designed the study, analyzed the results, and prepared the manuscript. M. H. characterized the CKII phosphorylations of lipin 1β and its peptides and identified the sites of phosphorylation. M. E. G. constructed the phosphorylation-deficient mutant forms of lipin 1β for expression and purification and performed the 14-3-3β interaction experiments with D. W. A. H. performed phosphorylation experiments on lipin 1β that included the phosphoamino acid analysis and the phosphopeptide mapping experiments. J. M. K. performed the PA phosphatase assays on lipin 1β phosphorylated by CKII.
This work was supported, in whole or in part, by National Institutes of Health Grants GM050679 (to G. M. C.) and DK101946 (to T. E. H.) from the United States Public Health Service. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PA
- phosphatidate
- CKI and CKII
- casein kinase I and II, respectively
- PVDF
- polyvinylidene difluoride
- PMSF
- phenylmethylsulfonyl fluoride
- TPCK
- l-1-tosylamido-2-phenylethyl chloromethyl ketone.
References
- 1. Brindley D. N., and Waggoner D. W. (1996) Phosphatidate phosphohydrolase and signal transduction. Chem. Phys. Lipids 80, 45–57 10.1016/0009-3084(96)02545-5 [DOI] [PubMed] [Google Scholar]
- 2. Vance D. E. (2004) Glycerolipid biosynthesis in eukaryotes. In Biochemistry of lipids, lipoproteins and membranes (Vance D. E., and Vance J., eds) 5th Ed, pp. 153–181, Elsevier Science Publishers B.V., Amsterdam [Google Scholar]
- 3. Carman G. M., and Han G.-S. (2009) Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 284, 2593–2597 10.1074/jbc.R800059200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reue K., and Brindley D. N. (2008) Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 49, 2493–2503 10.1194/jlr.R800019-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reue K. (2009) The lipin family: mutations and metabolism. Curr. Opin. Lipidol. 20, 165–170 10.1097/MOL.0b013e32832adee5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reue K., and Dwyer J. R. (2009) Lipin proteins and metabolic homeostasis. J. Lipid Res. 50, S109–S114 10.1194/jlr.R800052-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brindley D. N., Pilquil C., Sariahmetoglu M., and Reue K. (2009) Phosphatidate degradation: phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim. Biophys. Acta 1791, 956–961 10.1016/j.bbalip.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pascual F., and Carman G. M. (2013) Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim. Biophys. Acta 1831, 514–522 10.1016/j.bbalip.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vance J. E. (2018) Historical perspective: phosphatidylserine and phosphatidylethanolamine from the 1800s to the present. J. Lipid Res. 59, 923–944 10.1194/jlr.R084004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith S. W., Weiss S. B., and Kennedy E. P. (1957) The enzymatic dephosphorylation of phosphatidic acids. J. Biol. Chem. 228, 915–922 [PubMed] [Google Scholar]
- 11. Péterfy M., Phan J., Xu P., and Reue K. (2001) Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27, 121–124 10.1038/83685 [DOI] [PubMed] [Google Scholar]
- 12. Donkor J., Sariahmetoglu M., Dewald J., Brindley D. N., and Reue K. (2007) Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282, 3450–3457 [DOI] [PubMed] [Google Scholar]
- 13. Han G.-S., and Carman G. M. (2010) Characterization of the human LPIN1-encoded phosphatidate phosphatase isoforms. J. Biol. Chem. 285, 14628–14638 10.1074/jbc.M110.117747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collet J. F., Stroobant V., Pirard M., Delpierre G., and Van Schaftingen E. (1998) A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX(T/V) motif. J. Biol. Chem. 273, 14107–14112 10.1074/jbc.273.23.14107 [DOI] [PubMed] [Google Scholar]
- 15. Collet J. F., Stroobant V., and Van Schaftingen E. (1999) Mechanistic studies of ATPases. J. Biol. Chem. 274, 33985–33990 10.1074/jbc.274.48.33985 [DOI] [PubMed] [Google Scholar]
- 16. Han G.-S., Siniossoglou S., and Carman G. M. (2007) The cellular functions of the yeast lipin homolog Pah1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 282, 37026–37035 10.1074/jbc.M705777200 [DOI] [PubMed] [Google Scholar]
- 17. Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C. Jr, and Kelly D. P. (2006) Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 4, 199–210 10.1016/j.cmet.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 18. Zeharia A., Shaag A., Houtkooper R. H., Hindi T., de Lonlay P., Erez G., Hubert L., Saada A., de Keyzer Y., Eshel G., Vaz F. M., Pines O., and Elpeleg O. (2008) Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet. 83, 489–494 10.1016/j.ajhg.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang P., Verity M. A., and Reue K. (2014) Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab. 20, 267–279 10.1016/j.cmet.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nadra K., de Preux Charles A.-S., Médard J.-J., Hendriks W. T., Han G.-S., Grès S., Carman G. M., Saulnier-Blache J.-S., Verheijen M. H. G., and Chrast R. (2008) Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 22, 1647–1661 10.1101/gad.1638008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiedmann S., Fischer M., Koehler M., Neureuther K., Riegger G., Doering A., Schunkert H., Hengstenberg C., and Baessler A. (2008) Genetic variants within the LPIN1 gene, encoding lipin, are influencing phenotypes of the metabolic syndrome in humans. Diabetes 57, 209–217 10.2337/db07-0083 [DOI] [PubMed] [Google Scholar]
- 22. Phan J., and Reue K. (2005) Lipin, a lipodystrophy and obesity gene. Cell Metab. 1, 73–83 10.1016/j.cmet.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 23. Navratil A. R., Vozenilek A. E., Cardelli J. A., Green J. M., Thomas M. J., Sorci-Thomas M. G., Orr A. W., and Woolard M. D. (2015) Lipin-1 contributes to modified low-density lipoprotein-elicited macrophage pro-inflammatory responses. Atherosclerosis 242, 424–432 10.1016/j.atherosclerosis.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vozenilek A. E., Navratil A. R., Green J. M., Coleman D. T., Blackburn C. M. R., Finney A. C., Pearson B. H., Chrast R., Finck B. N., Klein R. L., Orr A. W., and Woolard M. D. (2018) Macrophage-associated lipin-1 enzymatic activity contributes to modified low-density lipoprotein-induced proinflammatory signaling and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 38, 324–334 10.1161/ATVBAHA.117.310455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Z., Gropler M. C., Mitra M. S., and Finck B. N. (2012) Complex interplay between the lipin 1 and the hepatocyte nuclear factor 4 α (HNF4α) pathways to regulate liver lipid metabolism. PLoS One 7, e51320 10.1371/journal.pone.0051320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J., Kim C., Jogasuria A., Han Y., Hu X., Wu J., Shen H., Chrast R., Finck B. N., and You M. (2016) Myeloid cell-specific lipin-1 deficiency stimulates endocrine adiponectin-FGF15 axis and ameliorates ethanol-induced liver injury in mice. Sci. Rep. 6, 34117 10.1038/srep34117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu M., Yin H., Mitra M. S., Liang X., Ajmo J. M., Nadra K., Chrast R., Finck B. N., and You M. (2013) Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology 58, 1953–1963 10.1002/hep.26589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen Z., Liang X., Rogers C. Q., Rideout D., and You M. (2010) Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G364–G374 10.1152/ajpgi.00456.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jang C. H., Kim K. M., Yang J. H., Cho S. S., Kim S. J., Shin S. M., Cho I. J., and Ki S. H. (2016) The role of lipin-1 in the regulation of fibrogenesis and TGF-β signaling in hepatic stellate cells. Toxicol. Sci. 153, 28–38 10.1093/toxsci/kfw109 [DOI] [PubMed] [Google Scholar]
- 30. Brohée L., Demine S., Willems J., Arnould T., Colige A. C., and Deroanne C. F. (2015) Lipin-1 regulates cancer cell phenotype and is a potential target to potentiate rapamycin treatment. Oncotarget 6, 11264–11280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He J., Zhang F., Tay L. W. R., Boroda S., Nian W., Levental K. R., Levental I., Harris T. E., Chang J. T., and Du G. (2017) Lipin-1 regulation of phospholipid synthesis maintains endoplasmic reticulum homeostasis and is critical for triple-negative breast cancer cell survival. FASEB J. 31, 2893–2904 10.1096/fj.201601353R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan X., Weng Y., Bai Y., Wang Z., Wang S., Zhu J., and Zhang F. (2018) Lipin-1 determines lung cancer cell survival and chemotherapy sensitivity by regulation of endoplasmic reticulum homeostasis and autophagy. Cancer Med. 7, 2541–2554 10.1002/cam4.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kourti M., Ikonomou G., Giakoumakis N. N., Rapsomaniki M. A., Landegren U., Siniossoglou S., Lygerou Z., Simos G., and Mylonis I. (2015) CK1δ restrains lipin-1 induction, lipid droplet formation and cell proliferation under hypoxia by reducing HIF-1α/ARNT complex formation. Cell. Signal. 27, 1129–1140 10.1016/j.cellsig.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meana C., García-Rostán G., Peña L., Lordén G., Cubero Á., Orduña A., Györffy B., Balsinde J., and Balboa M. A. (2018) The phosphatidic acid phosphatase lipin-1 facilitates inflammation-driven colon carcinogenesis. JCI Insight 3, 97506 10.1172/jci.insight.97506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferguson P. J., and El-Shanti H. I. (2007) Autoinflammatory bone disorders. Curr. Opin. Rheumatol. 19, 492–498 10.1097/BOR.0b013e32825f5492 [DOI] [PubMed] [Google Scholar]
- 36. Ferguson P. J., Chen S., Tayeh M. K., Ochoa L., Leal S. M., Pelet A., Munnich A., Lyonnet S., Majeed H. A., and El-Shanti H. (2005) Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J. Med. Genet. 42, 551–557 10.1136/jmg.2005.030759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aulchenko Y. S., Pullen J., Kloosterman W. P., Yazdanpanah M., Hofman A., Vaessen N., Snijders P. J., Zubakov D., Mackay I., Olavesen M., Sidhu B., Smith V. E., Carey A., Berezikov E., Uittenlinden A. G., et al. (2007) LPIN2 is associated with type 2 diabetes, glucose metabolism and body composition. Diabetes 56, 3020–3026 10.2337/db07-0338 [DOI] [PubMed] [Google Scholar]
- 38. Carman G. M., and Han G.-S. (2006) Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 31, 694–699 10.1016/j.tibs.2006.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reue K., and Zhang P. (2008) The lipin protein family: dual roles in lipid biosynthesis and gene expression. FEBS Lett. 582, 90–96 10.1016/j.febslet.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takeuchi K., and Reue K. (2009) Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296, E1195–E1209 10.1152/ajpendo.90958.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huffman T. A., Mothe-Satney I., and Lawrence J. C. Jr. (2002) Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc. Natl. Acad. Sci. U.S.A. 99, 1047–1052 10.1073/pnas.022634399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris T. E., Huffman T. A., Chi A., Shabanowitz J., Hunt D. F., Kumar A., and Lawrence J. C. Jr. (2007) Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J. Biol. Chem. 282, 277–286 10.1074/jbc.M609537200 [DOI] [PubMed] [Google Scholar]
- 43. Grimsey N., Han G.-S., O'Hara L., Rochford J. J., Carman G. M., and Siniossoglou S. (2008) Temporal and spatial regulation of the phosphatidate phosphatases lipin 1 and 2. J. Biol. Chem. 29166–29174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu G. H., and Gerace L. (2009) Sumoylation regulates nuclear localization of lipin-1alpha in neuronal cells. PLoS One 4, e7031 10.1371/journal.pone.0007031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li T. Y., Song L., Sun Y., Li J., Yi C., Lam S. M., Xu D., Zhou L., Li X., Yang Y., Zhang C. S., Xie C., Huang X., Shui G., Lin S. Y., et al. (2018) Tip60-mediated lipin 1 acetylation and ER translocation determine triacylglycerol synthesis rate. Nat. Commun. 9, 1916 10.1038/s41467-018-04363-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Péterfy M., Harris T. E., Fujita N., and Reue K. (2010) Insulin-stimulated interaction with 14-3-3 promotes cytoplasmic localization of lipin-1 in adipocytes. J. Biol. Chem. 285, 3857–3864 10.1074/jbc.M109.072488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peterson T. R., Sengupta S. S., Harris T. E., Carmack A. E., Kang S. A., Balderas E., Guertin D. A., Madden K. L., Carpenter A. E., Finck B. N., and Sabatini D. M. (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 10.1016/j.cell.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zanivan S., Gnad F., Wickström S. A., Geiger T., Macek B., Cox J., Fässler R., and Mann M. (2008) Solid tumor proteome and phosphoproteome analysis by high resolution mass spectrometry. J. Proteome Res. 7, 5314–5326 10.1021/pr800599n [DOI] [PubMed] [Google Scholar]
- 49. Grimsrud P. A., Carson J. J., Hebert A. S., Hubler S. L., Niemi N. M., Bailey D. J., Jochem A., Stapleton D. S., Keller M. P., Westphall M. S., Yandell B. S., Attie A. D., Coon J. J., and Pagliarini D. J. (2012) A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Cell Metab. 16, 672–683 10.1016/j.cmet.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Humphrey S. J., Yang G., Yang P., Fazakerley D. J., Stöckli J., Yang J. Y., and James D. E. (2013) Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 17, 1009–1020 10.1016/j.cmet.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lundby A., Andersen M. N., Steffensen A. B., Horn H., Kelstrup C. D., Francavilla C., Jensen L. J., Schmitt N., Thomsen M. B., and Olsen J. V. (2013) In vivo phosphoproteomics analysis reveals the cardiac targets of β-adrenergic receptor signaling. Sci. Signal. 6, rs11 [DOI] [PubMed] [Google Scholar]
- 52. Shimizu K., Fukushima H., Ogura K., Lien E. C., Nihira N. T., Zhang J., North B. J., Guo A., Nagashima K., Nakagawa T., Hoshikawa S., Watahiki A., Okabe K., Yamada A., Toker A., et al. (2017) The SCFβ-TRCP E3 ubiquitin ligase complex targets Lipin1 for ubiquitination and degradation to promote hepatic lipogenesis. Sci. Signal. 10, eaah4117 10.1126/scisignal.aah4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brognard J., and Hunter T. (2011) Protein kinase signaling networks in cancer. Curr. Opin. Genet. Dev. 21, 4–11 10.1016/j.gde.2010.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pinna L. A., and Meggio F. (1997) Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 3, 77–97 [DOI] [PubMed] [Google Scholar]
- 55. Faust M., and Montenarh M. (2000) Subcellular localization of protein kinase CK2: a key to its function? Cell Tissue Res. 301, 329–340 10.1007/s004410000256 [DOI] [PubMed] [Google Scholar]
- 56. Litchfield D. W. (2003) Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369, 1–15 10.1042/bj20021469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blom N., Sicheritz-Pontén T., Gupta R., Gammeltoft S., and Brunak S. (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649 10.1002/pmic.200300771 [DOI] [PubMed] [Google Scholar]
- 58. Granade M. E., and Harris T. E. (2018) Purification of lipin and measurement of phosphatidic acid phosphatase activity from liposomes. Methods Enzymol. 607, 373–388 10.1016/bs.mie.2018.04.028 [DOI] [PubMed] [Google Scholar]
- 59. Prasad T. S., Kandasamy K., and Pandey A. (2009) Human Protein Reference Database and Human Proteinpedia as discovery tools for systems biology. Methods Mol. Biol. 577, 67–79 10.1007/978-1-60761-232-2_6 [DOI] [PubMed] [Google Scholar]
- 60. Wilkins M. R., Gasteiger E., Bairoch A., Sanchez J. C., Williams K. L., Appel R. D., and Hochstrasser D. F. (1999) Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 112, 531–552 [DOI] [PubMed] [Google Scholar]
- 61. Csaki L. S., Dwyer J. R., Fong L. G., Tontonoz P., Young S. G., and Reue K. (2013) Lipins, lipinopathies, and the modulation of cellular lipid storage and signaling. Prog. Lipid Res. 52, 305–316 10.1016/j.plipres.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reue K., and Donkor J. (2007) Genetic factors in type 2 diabetes: all in the (lipin) family. Diabetes 56, 2842–2843 10.2337/db07-1288 [DOI] [PubMed] [Google Scholar]
- 63. Carman G. M. (2018) Discoveries of the phosphatidate phosphatase genes in yeast published in the Journal of Biological Chemistry. J. Biol. Chem. (2019) 294, 1681–1689 10.1074/jbc.TM118.004159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Hara L., Han G.-S., Peak-Chew S., Grimsey N., Carman G. M., and Siniossoglou S. (2006) Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 281, 34537–34548 10.1074/jbc.M606654200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gruhler A., Olsen J. V., Mohammed S., Mortensen P., Faergeman N. J., Mann M., and Jensen O. N. (2005) Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol. Cell Proteomics 4, 310–327 10.1074/mcp.M400219-MCP200 [DOI] [PubMed] [Google Scholar]
- 66. Li X., Gerber S. A., Rudner A. D., Beausoleil S. A., Haas W., Villén J., Elias J. E., and Gygi S. P. (2007) Large-scale phosphorylation analysis of α-factor-arrested Saccharomyces cerevisiae. J. Proteome. Res. 6, 1190–1197 10.1021/pr060559j [DOI] [PubMed] [Google Scholar]
- 67. Chi A., Huttenhower C., Geer L. Y., Coon J. J., Syka J. E., Bai D. L., Shabanowitz J., Burke D. J., Troyanskaya O. G., and Hunt D. F. (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 104, 2193–2198 10.1073/pnas.0607084104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smolka M. B., Albuquerque C. P., Chen S. H., and Zhou H. (2007) Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 10.1073/pnas.0701622104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Albuquerque C. P., Smolka M. B., Payne S. H., Bafna V., Eng J., and Zhou H. (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell Proteomics 7, 1389–1396 10.1074/mcp.M700468-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Soufi B., Kelstrup C. D., Stoehr G., Fr−ohlich F., Walther T. C., and Olsen J. V. (2009) Global analysis of the yeast osmotic stress response by quantitative proteomics. Mol. Biosyst. 5, 1337–1346 10.1039/b902256b [DOI] [PubMed] [Google Scholar]
- 71. Gnad F., de Godoy L. M., Cox J., Neuhauser N., Ren S., Olsen J. V., and Mann M. (2009) High-accuracy identification and bioinformatic analysis of in vivo protein phosphorylation sites in yeast. Proteomics 9, 4642–4652 10.1002/pmic.200900144 [DOI] [PubMed] [Google Scholar]
- 72. Helbig A. O., Rosati S., Pijnappel P. W., van Breukelen B., Timmers M. H., Mohammed S., Slijper M., and Heck A. J. (2010) Perturbation of the yeast N-acetyltransferase NatB induces elevation of protein phosphorylation levels. BMC. Genomics 11, 685 10.1186/1471-2164-11-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Soulard A., Cremonesi A., Moes S., Schütz F., Jenö P., and Hall M. N. (2010) The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol. Biol. Cell 21, 3475–3486 10.1091/mbc.e10-03-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bodenmiller B., Wanka S., Kraft C., Urban J., Campbell D., Pedrioli P. G., Gerrits B., Picotti P., Lam H., Vitek O., Brusniak M. Y., Roschitzki B., Zhang C., Shokat K. M., Schlapbach R., et al. (2010) Phosphoproteomic analysis reveals interconnected system-wide responses to perturbations of kinases and phosphatases in yeast. Sci. Signal. 3, rs4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Swaney D. L., Beltrao P., Starita L., Guo A., Rush J., Fields S., Krogan N. J., and Villén J. (2013) Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 10, 676–682 10.1038/nmeth.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Su W.-M., Han G.-S., Casciano J., and Carman G. M. (2012) Protein kinase A-mediated phosphorylation of Pah1p phosphatidate phosphatase functions in conjunction with the Pho85p-Pho80p and Cdc28p-cyclin B kinases to regulate lipid synthesis in yeast. J. Biol. Chem. 287, 33364–33376 10.1074/jbc.M112.402339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Su W.-M., Han G.-S., and Carman G. M. (2014) Cross-talk phosphorylations by protein kinase C and Pho85p-Pho80p protein kinase regulate Pah1p phosphatidate phosphatase abundance in Saccharomyces cerevisiae. J. Biol. Chem. 289, 18818–18830 10.1074/jbc.M114.581462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Choi H.-S., Su W.-M., Morgan J. M., Han G.-S., Xu Z., Karanasios E., Siniossoglou S., and Carman G. M. (2011) Phosphorylation of phosphatidate phosphatase regulates its membrane association and physiological functions in Saccharomyces cerevisiae: identification of Ser602, Thr723, and Ser744 as the sites phosphorylated by CDC28 (CDK1)-encoded cyclin-dependent kinase. J. Biol. Chem. 286, 1486–1498 10.1074/jbc.M110.155598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Choi H.-S., Su W.-M., Han G.-S., Plote D., Xu Z., and Carman G. M. (2012) Pho85p-Pho80p phosphorylation of yeast Pah1p phosphatidate phosphatase regulates its activity, location, abundance, and function in lipid metabolism. J. Biol. Chem. 287, 11290–11301 10.1074/jbc.M112.346023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hsieh L.-S., Su W.-M., Han G.-S., and Carman G. M. (2016) Phosphorylation of yeast Pah1 phosphatidate phosphatase by casein kinase II regulates its function in lipid metabolism. J. Biol. Chem. 291, 9974–9990 10.1074/jbc.M116.726588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Santos-Rosa H., Leung J., Grimsey N., Peak-Chew S., and Siniossoglou S. (2005) The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24, 1931–1941 10.1038/sj.emboj.7600672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hsieh L.-S., Su W.-M., Han G.-S., and Carman G. M. (2015) Phosphorylation regulates the ubiquitin-independent degradation of yeast Pah1 phosphatidate phosphatase by the 20S proteasome. J. Biol. Chem. 290, 11467–11478 10.1074/jbc.M115.648659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glover C. V., 3rd (1998) On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 59, 95–133 [DOI] [PubMed] [Google Scholar]
- 84. Poole A., Poore T., Bandhakavi S., McCann R. O., Hanna D. E., and Glover C. V. (2005) A global view of CK2 function and regulation. Mol. Cell Biochem. 274, 163–170 10.1007/s11010-005-2945-z [DOI] [PubMed] [Google Scholar]
- 85. Heesom K. J., Avison M. B., Diggle T. A., and Denton R. M. (1998) Insulin-stimulated kinase from rat fat cells that phosphorylates initiation factor 4E-binding protein 1 on the rapamycin-insensitive site (serine-111). Biochem. J. 336, 39–48 10.1042/bj3360039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Diggle T. A., Schmitz-Peiffer C., Borthwick A. C., Welsh G. I., and Denton R. M. (1991) Evidence that insulin activates casein kinase 2 in rat epididymal fat-cells and that this may result in the increased phosphorylation of an acid-soluble 22 kDa protein. Biochem. J. 279, 545–551 10.1042/bj2790545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Witters L. A., Tipper J. P., and Bacon G. W. (1983) Stimulation of site-specific phosphorylation of acetyl coenzyme A carboxylase by insulin and epinephrine. J. Biol. Chem. 258, 5643–5648 [PubMed] [Google Scholar]
- 88. Kim K.-H. (1997) Regulation of mammalian acetyl-coenzyme A carboxylase. Annu. Rev. Nutr. 17, 77–99 10.1146/annurev.nutr.17.1.77 [DOI] [PubMed] [Google Scholar]
- 89. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 90. Boyle W. J., van der Geer P., and Hunter T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201, 110–149 10.1016/0076-6879(91)01013-R [DOI] [PubMed] [Google Scholar]
- 91. Yang W.-L., and Carman G. M. (1995) Phosphorylation of CTP synthetase from Saccharomyces cerevisiae by protein kinase C. J. Biol. Chem. 270, 14983–14988 10.1074/jbc.270.25.14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. MacDonald J. I. S., and Kent C. (1994) Identification of phosphorylation sites in rat liver CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 269, 10529–10537 [PubMed] [Google Scholar]
- 93. Burnette W. (1981) Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112, 195–203 10.1016/0003-2697(81)90281-5 [DOI] [PubMed] [Google Scholar]
- 94. Boroda S., Takkellapati S., Lawrence R. T., Entwisle S. W., Pearson J. M., Granade M. E., Mullins G. R., Eaton J. M., Villén J., and Harris T. E. (2017) The phosphatidic acid-binding, polybasic domain is responsible for the differences in the phosphoregulation of lipins 1 and 3. J. Biol. Chem. 292, 20481–20493 10.1074/jbc.M117.786574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Havriluk T., Lozy F., Siniossoglou S., and Carman G. M. (2008) Colorimetric determination of pure Mg2+-dependent phosphatidate phosphatase activity. Anal. Biochem. 373, 392–394 10.1016/j.ab.2007.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liao M. J., and Prestegard J. H. (1979) Fusion of phosphatidic acid-phosphatidylcholine mixed lipid vesicles. Biochim. Biophys. Acta 550, 157–173 10.1016/0005-2736(79)90204-9 [DOI] [PubMed] [Google Scholar]
- 97. Koter M., de Kruijff B., and van Deenen L. L. (1978) Calcium-induced aggregation and fusion of mixed phosphatidylcholine-phosphatidic acid vesicles as studied by 31P NMR. Biochim. Biophys. Acta 514, 255–263 10.1016/0005-2736(78)90296-1 [DOI] [PubMed] [Google Scholar]
- 98. Blackwood R. A., Smolen J. E., Transue A., Hessler R. J., Harsh D. M., Brower R. C., and French S. (1997) Phospholipase D activity facilitates Ca2+-induced aggregation and fusion of complex liposomes. Am. J. Physiol. 272, C1279–C1285 10.1152/ajpcell.1997.272.4.C1279 [DOI] [PubMed] [Google Scholar]
- 99. Weigert R., Silletta M. G., Spanò S., Turacchio G., Cericola C., Colanzi A., Senatore S., Mancini R., Polishchuk E. V., Salmona M., Facchiano F., Burger K. N., Mironov A., Luini A., and Corda D. (1999) CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402, 429–433 10.1038/46587 [DOI] [PubMed] [Google Scholar]
- 100. Goñi F. M., and Alonso A. (1999) Structure and functional properties of diacylglycerols in membranes. Prog. Lipid Res. 38, 1–48 10.1016/S0163-7827(98)00021-6 [DOI] [PubMed] [Google Scholar]
- 101. Chernomordik L., Kozlov M. M., and Zimmerberg J. (1995) Lipids in biological membrane fusion. J. Membr. Biol. 146, 1–14 [DOI] [PubMed] [Google Scholar]
- 102. Roth M. G. (2008) Molecular mechanisms of PLD function in membrane traffic. Traffic 9, 1233–1239 10.1111/j.1600-0854.2008.00742.x [DOI] [PubMed] [Google Scholar]
- 103. Morris A. J. (2007) Regulation of phospholipase D activity, membrane targeting and intracellular trafficking by phosphoinositides. Biochem. Soc. Symp. 247–257 10.1042/BSS0740247 [DOI] [PubMed] [Google Scholar]
- 104. Maissel A., Marom M., Shtutman M., Shahaf G., and Livneh E. (2006) PKCη is localized in the Golgi, ER and nuclear envelope and translocates to the nuclear envelope upon PMA activation and serum-starvation: C1b domain and the pseudosubstrate containing fragment target PKCη to the Golgi and the nuclear envelope. Cell. Signal. 18, 1127–1139 10.1016/j.cellsig.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 105. Lehel C., Oláh Z., Jakab G., Szállási Z., Petrovics G., Harta G., Blumberg P. M., and Anderson W. B. (1995) Protein kinase C ϵ subcellular localization domains and proteolytic degradation sites: a model for protein kinase C conformational changes. J. Biol. Chem. 270, 19651–19658 10.1074/jbc.270.33.19651 [DOI] [PubMed] [Google Scholar]
- 106. Baron C. L., and Malhotra V. (2002) Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325–328 10.1126/science.1066759 [DOI] [PubMed] [Google Scholar]


