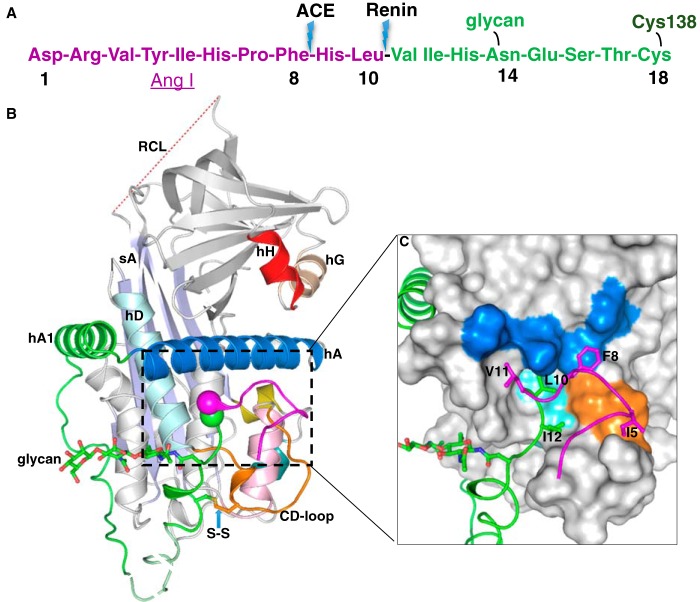
Figure 1.
The crystal structure of human glycosylated AGT. A, the N-terminal tail sequence of AGT, indicating the renin and ACE cleavage sites, the glycosylation site, and the conserved disulfide bond. B, the structure of AGT is shown as a cartoon. The serpin template is in gray, and helix A (hA) is in marine with the A-sheet (sA) in light blue and the disordered RCL in red dashes. The Ang I segment is in magenta, and the following amino-tail is in green with the scissile bond shown as magenta and green spheres. Cysteine 18 in the amino tail forms a disulfide bond with cysteine 138 of the CD-loop (brown). The glycan attached to Asn14 is shown as green sticks. The segment from Glu20 to Pro29 (dashed, pale green) is disordered in the structure and modeled for illustration. Helix H (hH) is in red, and helix G (hG) is in wheat color. C, surface representation of the main body of AGT, with the extra N terminus (residues 1–63) shown in a cartoon representation. The Ang I peptide is mainly stabilized by hydrophobic interactions with the main body. Residues Ile5, Phe8, Leu10, Val11, and Ile12 (shown as sticks) form hydrophobic interactions with residues in the CD-loop (Val131, Pro132, and Trp133 as brown surface), helix A (Leu68, Met72, Leu76, and Phe79 as marine surface), and helix D (hD; Leu142 and Val147 as cyan surface). The scissile bond (Leu10–Val11) is buried in the hydrophobic cavity.