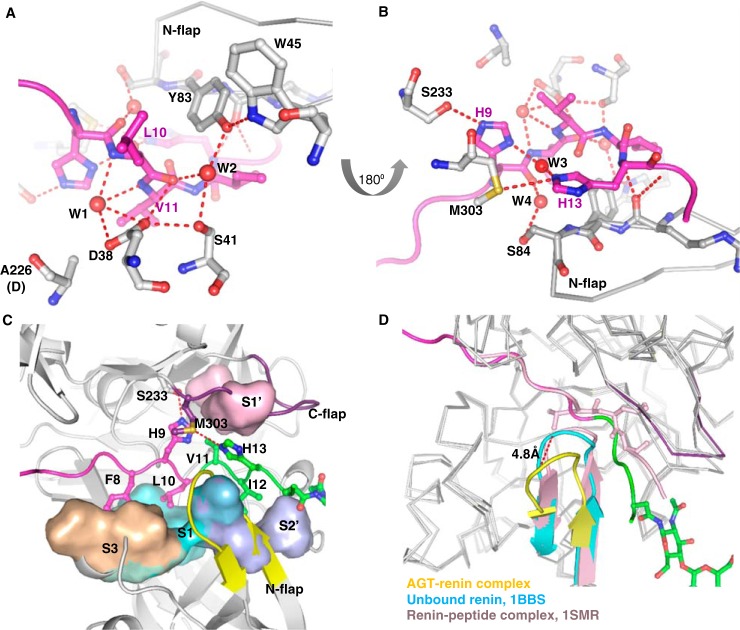
Figure 3.
Interactions inside the renin active cleft. A, renin residues are shown as gray, and the AGT peptide is in magenta. Asp38 in renin is hydrogen-bonded to the carbonyl oxygen of the scissile bond and a water (W1) that is also within hydrogen-bond range to the other mutated aspartic acid (Ala226). The conserved hydrogen bond network Trp45-Tyr83-water (W2)-Ser41-Asp38 is present. B, His9 in AGT is hydrogen-bonded to Ser233. His9 also connects to His13 through a bridging water (W3). Ser84 in the N-flap forms a hydrogen bond with a water (W4), which connects to the carbonyl oxygen of His9. C, binding subsites in the renin active cleft. Subsite S3 (Pro118, Phe119, Leu121, Ala122, and Phe124 of renin) accommodates Phe8; S1 (Phe119, Phe124, Val127, Val36, and Tyr83) accommodates Leu10; S1′ (Leu224 and Ile305) accommodates Val11; and S2′ (Ile137, Leu81, and Tyr83) accommodates Ile12. The N-flap of renin is shown as yellow, and the C-flap is in purple. D, the N-flap of renin (yellow) in the AGT–renin complex adopts an “open” position with a movement of 4.8 Å compared with its conformation in the unbound renin (cyan, PDB code 1BBS). There is no such conformational change when a decapeptide substrate (corresponding to residues 4–14 of rat AGT) is bound to mouse submaxillary renin (pink; PDB entry 1SMR). The N-terminal residues 1–10 of the AGT peptide are in magenta and the following residues 11–15 and the glycan are shown as green.