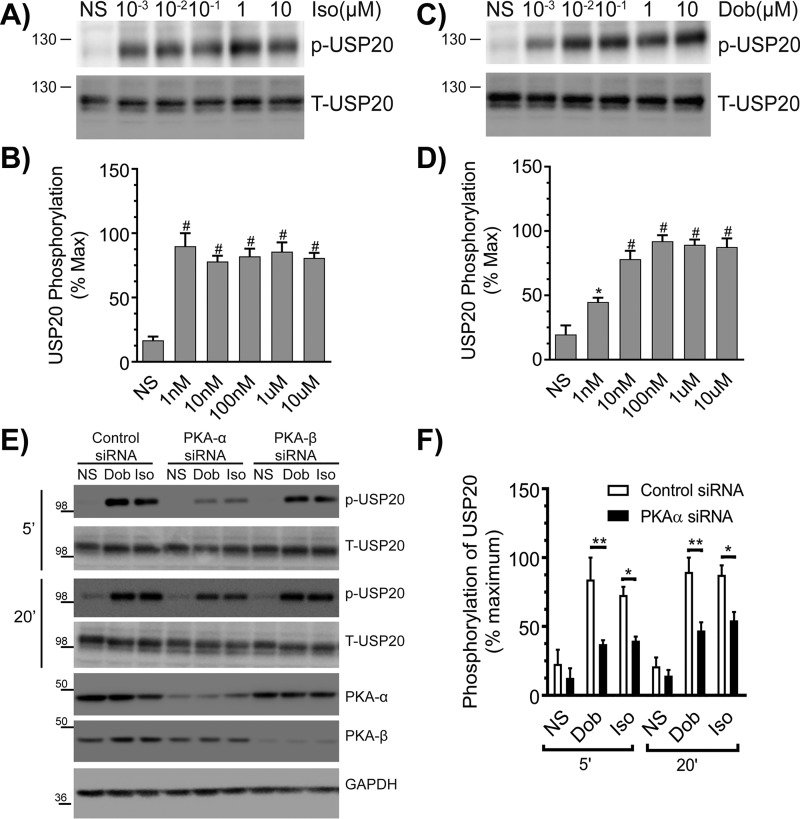
Figure 3.
β1AR activation promotes phosphorylation of USP20 on serine 333 by PKAα. A, HEK-293 cells stably expressing FLAG-β1AR were stimulated for 5 min with indicated amounts of Iso, and equivalent cell extracts were analyzed for USP20 phosphorylation (p-USP20) and expression (T-USP20). B, phospho-USP20 bands were normalized to total USP20 in each sample and plotted as % maximum signal showing means ± S.E. from n = 3 independent experiments. # denotes p < 0.01 compared with nonstimulated (NS) samples, one-way ANOVA, Bonferroni's post-test. C and D, experiments and analyses were as in A and B, except that the β1AR-selective agonist dobutamine was used. #, p < 0.05; *, p < 0.01 compared with nonstimulated. E, HEK-293 cells stably expressing FLAG-β1AR were transiently transfected with siRNAs targeting no mRNA (control), PKAα, or PKAβ for 48 h, serum-starved for 60 min, and then stimulated with 1 μm Dob or 1 μm Iso for 5 or 20 min. Cell lysates were immunoblotted for p-USP20, t-USP20, PKAα, PKAβ, and GAPDH. F, quantification of p-USP20 normalized to t-USP20. n = 3, *, p < 0.01; **, p < 0.001, ANOVA, Bonferroni's post-test.