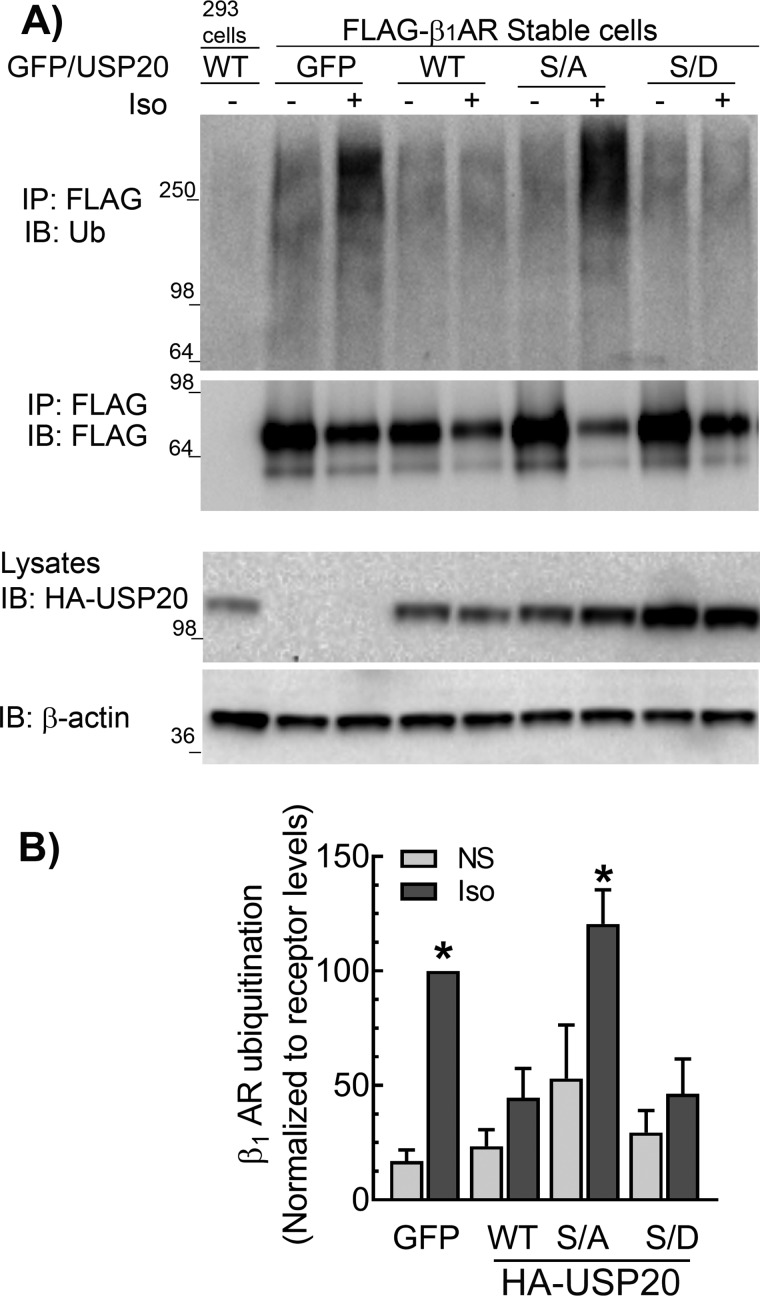
Figure 5.
Ser-333 phosphorylation preserves DUB activity of USP20 facilitating β1AR deubiquitination. A, HEK-293 cells stably expressing FLAG-β1AR were infected at equal m.o.i. with recombinant adenoviruses encoding eGFP or HA-tagged USP20 WT, S333A (S/A), or S333D (S/D) constructs and stimulated with 1 μm Iso for 60 min. The receptors were isolated with M2 anti-FLAG affinity gel. The immunoprecipitates (IP) were separated by SDS-PAGE and immunoblotted (IB) with antibodies specific to ubiquitin (rabbit polyclonal, Bethyl Laboratories Inc.) and FLAG antibodies as in Fig. 1A. Bottom panels show the expression levels of USP20 WT or mutants in the lysates as detected by monoclonal anti-HA (12CA5) antibody and detection of β-actin. B, ubiquitin smears in each lane from the IP blot in A were quantified, normalized to β1AR signals, and plotted as bars. *, p < 0.05 versus GFP(NS), WT(NS and Iso), and S/D (NS and Iso); two-way ANOVA Holm-Sidak's post-test. NS, not stimulated. Data are means ± S.E. of four independent experiments.