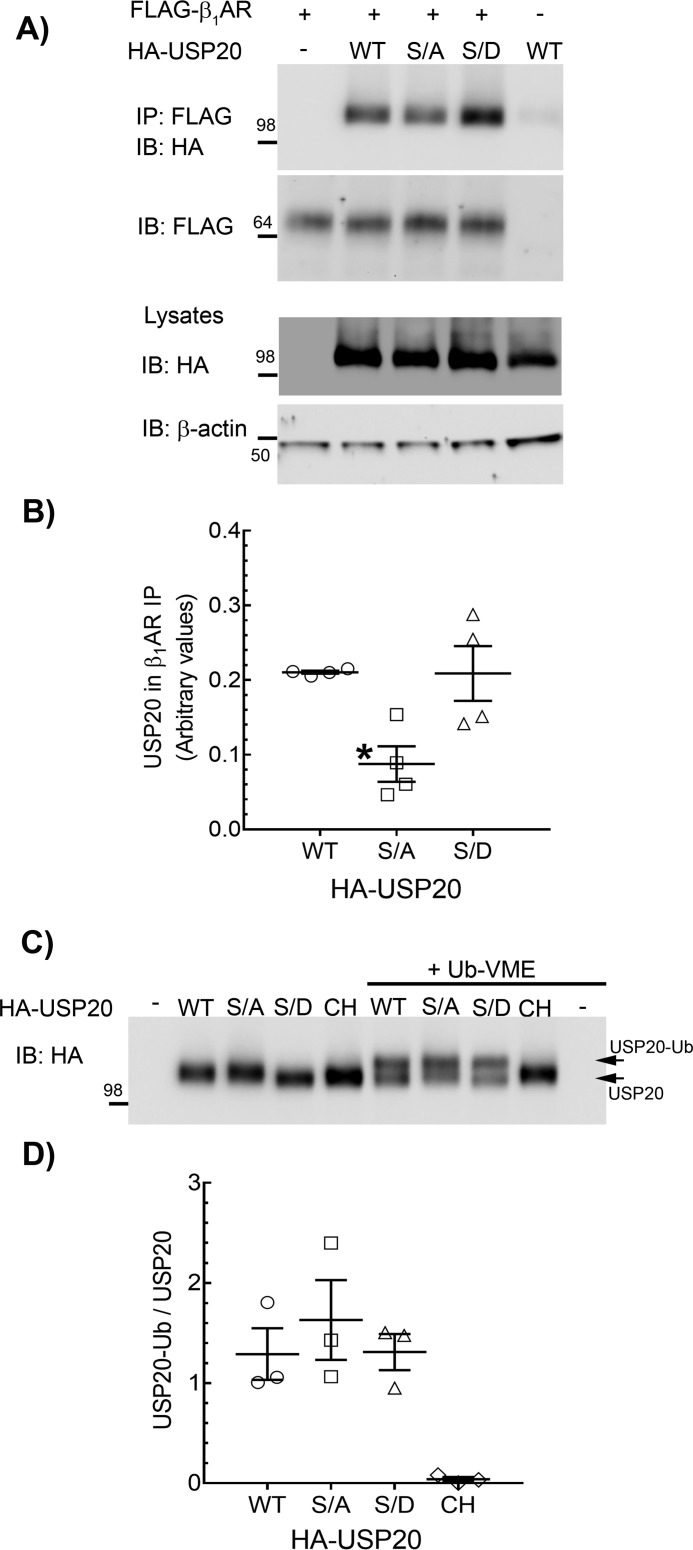
Figure 7.
Binding properties and DUB activity of USP20 phospho-mutants. A, HEK-293 cells stably expressing FLAG-β1AR were infected at equal m.o.i. with recombinant adenoviruses encoding eGFP or HA-tagged USP20 WT, S333A, or S333D constructs, and the receptors were isolated with M2 anti-FLAG affinity gel. The immunoprecipitates (IP) were separated by SDS-PAGE and immunoblotted (IB) with anti-HA (rabbit polyclonal, Cell Signaling Technology) and anti-FLAG antibodies. Lysates were serially immunoblotted for HA and β-actin. B, quantification of USP20 in receptor IPs from four independent experiments is plotted as USP20/β1AR ratio. *, p < 0.05; versus WT and S/D, one-way ANOVA, Bonferroni's test. C, expression of HA-tagged USP20 WT, S333A, S333D, or Cys-His were induced in HEK-293 cells by respective recombinant adenoviral infections. Lysates that contain ∼ 4 pmol of each USP20 (see “Experimental procedures”) were incubated with buffer or 20 pmol of ubiquitin-VME in vitro at 37 °C. Unmodified USP20 and USP20 that is covalently linked with ubiquitin-VME were detected by immunoblotting with rabbit polyclonal anti-HA antibody (Cell Signaling Technology). D, ratios of USP20-Ub and unmodified USP20 for each construct are plotted. No significant difference between WT, S/A, and S/D (one-way ANOVA, Bonferroni's test).