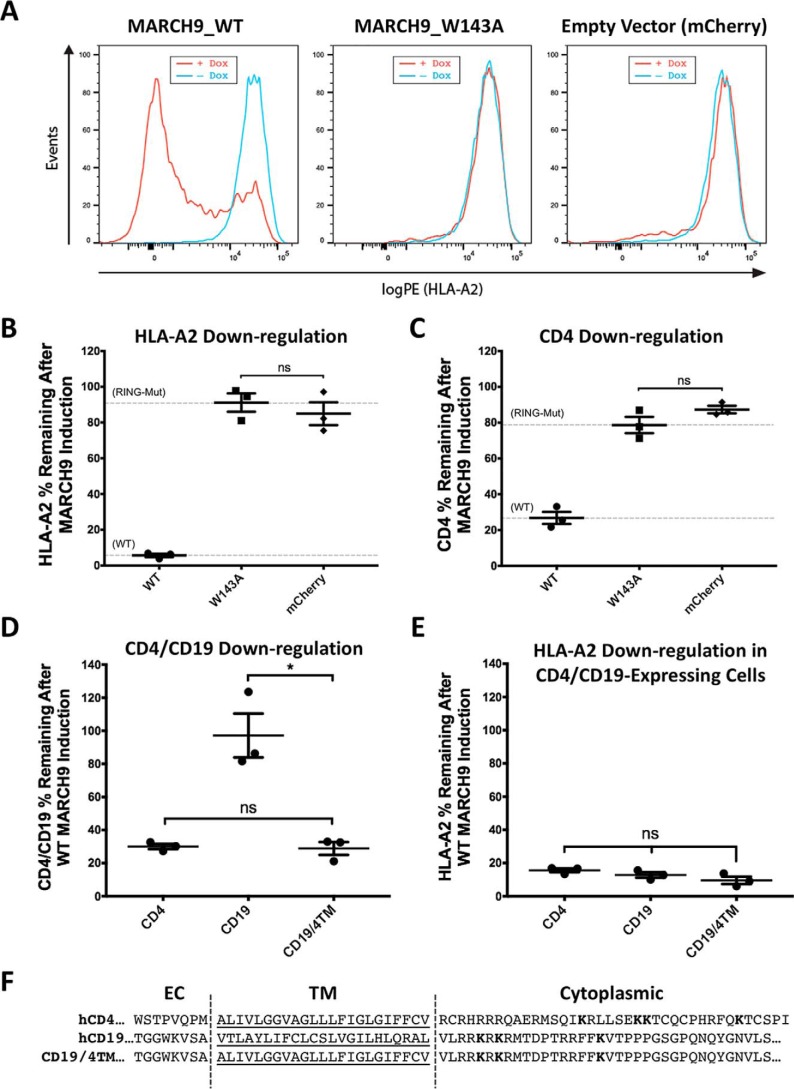
Figure 1.
Susceptibility to MARCH9-mediated down-regulation can be transferred by the CD4 TM domain sequence. A, representative histograms showing 293T cells transduced with lentivirus-encoding dox-inducible WT (left), W143A RING mutant (middle), or no MARCH9 (right) with a constitutively expressed mCherry marker. Transduced cells were incubated with or without dox for 48 h, and surface levels of endogenous HLA-A2 on mCherry+ cells were measured by flow cytometry. B and C, 293T cells stably expressing human CD4 were transduced with lentiviral constructs shown in A, and surface levels of HLA-A2 in B and CD4 in C were measured on mCherry+ cells after 48 h of culture with or without dox. Data are presented as percentage of substrate remaining on the surface of dox-treated cells relative to untreated cells from the same transduction. Dashed lines indicate mean substrate level remaining on dox-treated cells expressing control WT (lower) and RING mutant W143A (upper) MARCH9. D and E, 293T cells stably expressing human CD4, human CD19, or a chimeric CD19 protein containing the TM domain of CD4 (CD19–CD4TM) were transduced with WT MARCH9 and cultured with and without doxycycline for 48 h. Surface levels of CD4/CD19 (D) and HLA-A2 (E) were measured on mCherry+ cells by flow cytometry and presented as in B and C. In all graphs, lines represent the mean and S.E. of three independent experiments performed on different days (n = 3), and each point represents data from one experiment. Unpaired t test: ns, not significant; **, p < 0.02. F, human CD4 and CD19 partial amino acid sequences showing the residues that were swapped in the CD19–CD4TM chimera. Predicted TM domains underlined. Lysine residues in cytoplasmic tails highlighted in bold represent possible ubiquitination sites.