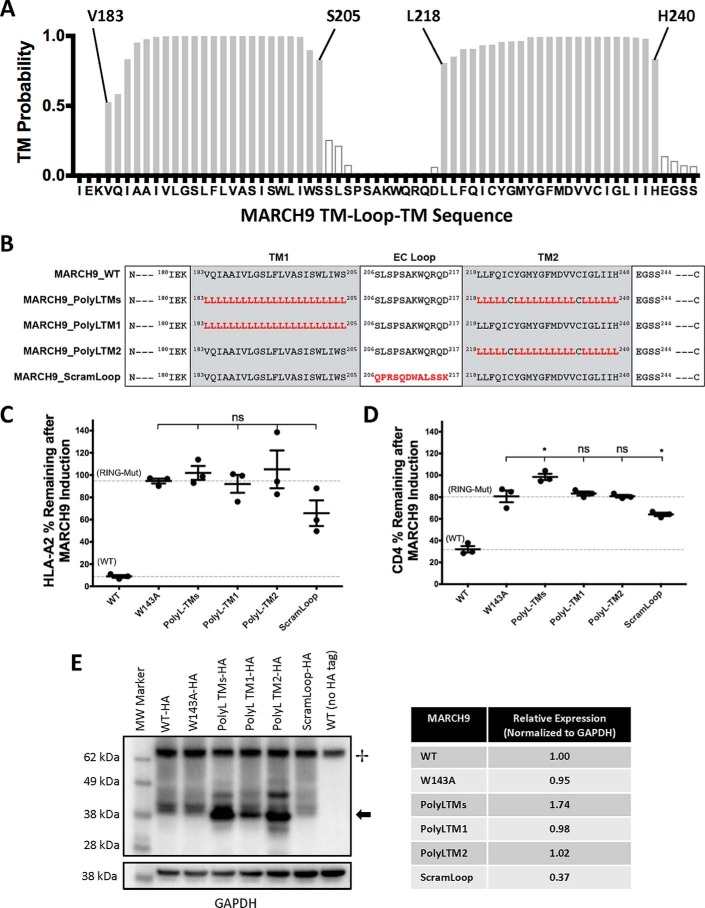
Figure 2.
MARCH9 TM sequences contain features that are absolutely required for substrate down-regulation. A, TMHMM (46) prediction of the locations of two TM domains in human MARCH9. Shaded bars are 50% confidence or better, and boundary residues for TM1 (Val-183–Ser-205) and TM2 (Leu-218–His-240) are marked above the graph. B, sequences of mutants analyzed in this figure. Red bold font indicates substituted amino acids. Two cysteines in predicted TM2 were not substituted. MARCH9_ScramLoop was generated by randomizing the 12-amino acid loop sequence such that no position contained the native amino acid but the overall amino acid content was unchanged. C and D, 293T cells stably expressing human CD4 were transduced with the indicated lentiviral constructs, and surface levels of HLA-A2 (C) and CD4 (D) were measured after 48 h culture with or without dox as in Fig. 1. Dashed lines indicate mean substrate level remaining on dox-treated cells expressing control WT (lower) and RING mutant W143A (upper) MARCH9. Unpaired t test: ns, not significant; *, p < 0.05. E, Western blot analysis of MARCH9 expression. WT and mutant MARCH9 sequences with C-terminal HA tag were transiently expressed in 293T cells under the strong EF1α promoter by calcium phosphate transfection and harvested 24–48 h later. Whole-cell lysates were separated by reducing SDS-PAGE, transferred to PVDF, and sequentially immunoblotted using α-GAPDH and α-HA antibodies. + marks the positions of a cellular product detected by SA-HRP; arrow marks the position of the HA-tagged MARCH9 protein. Table shows expression normalized to GAPDH loading control and relative to MARCH9-WT, quantitated by densitometry.