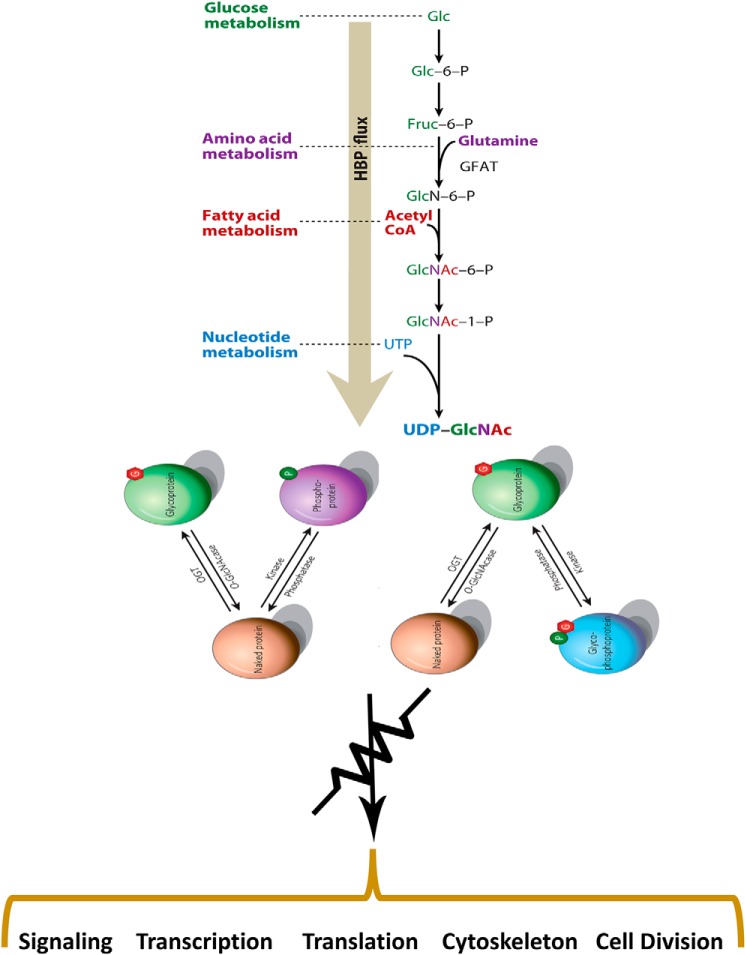
Figure 1.
The HBP links flux through major metabolic pathways, allowing O-GlcNAcylation to serve as a “rheostat” that modulates most cellular processes in response to nutrients. The biosynthesis of UDP-GlcNAc, the donor for the OGT, is directly coupled to flux through glucose, amino acid, fatty acid, and nucleotide metabolic pathways. OGT is highly sensitive to UDP-GlcNAc concentrations, both in terms of activity and selectivity. O-GlcNAcylation has extensive cross-talk with phosphorylation. Shown is the universal symbol for a rheostat, indicating that unlike phosphorylation, which is more analogous to a switch, O-GlcNAc serves in a more analog fashion as a rheostat to modulate processes in response to nutrients and stress. GFAT, glutamine:fructose-6-phosphate amidotransferase. Modified from Refs. 63 and 341. This research was originally published in Annual Review of Biochemistry. Hart, G. W., Slawson, C., Ramirez-Correa, G., and Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011; 80:825–858 © Annual Reviews and Nature. Hart, G. W., Housley, M. P., and Slawson, C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007; 446:1017–1022 © Springer Nature.