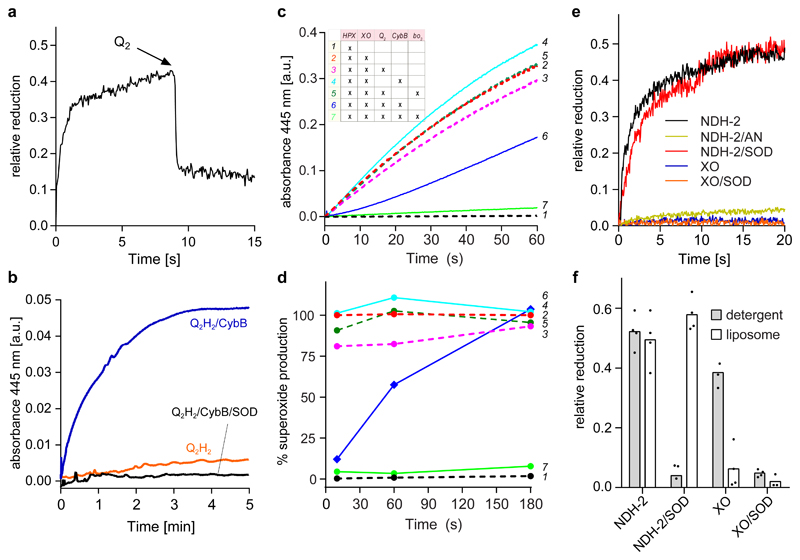
Fig. 2. CybB shows superoxide-oxidase activity in vitro.
(a) CybB was pre-reduced with HPX/XO before oxidized ubiquinone Q2 was added. The kinetic trace shown is a representative of at least 3 independent measurements. (b) Superoxide production by ubiquinol Q2H2 and CybB. The two components were mixed in the absence (blue) or in the presence (black) of SOD under aerobic conditions. Superoxide production was monitored spectrophotometrically at 445 nm following the conversion of a tetrazolium salt (WST-1) to formazan. The kinetic traces shown are representative of at least 3 independent measurements. (c) Superoxide production by HPX/XO and its suppression by CybB. See inset for the different combinations and text for further details. The kinetic traces shown are representative of at least 3 independent measurements. (d) Relative superoxide production rates at different time points during the experiments of Fig. 2c. The data are normalized to the rate of superoxide production by NADH/NDH-2 at each respective time point. (e) Membrane embedded CybB reacts preferentially with membrane derived superoxide. CybB was reconstituted into liposomes from E. coli polar lipid extract and mixed with superoxide produced either by NADH/NDH-2 or HPX/XO and either in the presence or absence of SOD under aerobic conditions. No reduction of CybB by NADH/NDH-2 was observed under anaerobic (AN) conditions. The kinetic traces shown are representative of at least 3 independent measurements. (f) Comparison of the results from Fig. 2e (open bars) with similar experiments performed with detergent solubilized CybB (grey bars). Mean values and the individual data points from n≥3 biologically independent experiments are shown.