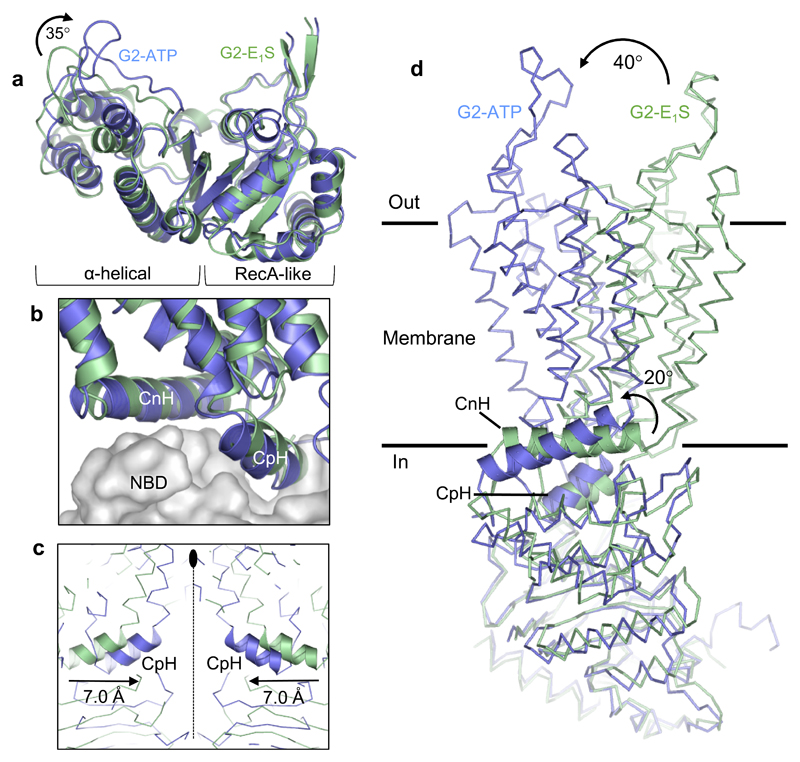
Figure 3. ATP-induced conformational changes.
a, Superposition of the RecA-like subdomains of the NBDs of the ABCG2EQ-E1S (green) and ABCG2EQ-ATP (blue) structures. A ~35° inward rotation of the helical subdomain is observed upon ATP binding. b, Superposition of one ABCG2 monomer of the ABCG2EQ-E1S and ABCG2EQ-ATP structures, with the NBDs shown as grey surface and the TMDs as ribbons. The interface helices, CpH and CnH, are labeled. c, Superposition of the ABCG2EQ-E1S and ABCG2EQ-ATP structures along the 2-fold symmetry axis (dotted line), showing a 7 Å inward movement of the CpH’s of each ABCG2 monomer. d, Comparison of a single ABCG2 monomer of the ABCG2EQ-E1S and ABCG2EQ-ATP structures. The NBDs have been superimposed, revealing a 20° rotation of the CnH and CpH (shown as ribbons), as well as a 40° rotation of the TMDs relative to one another.