Figure 4.
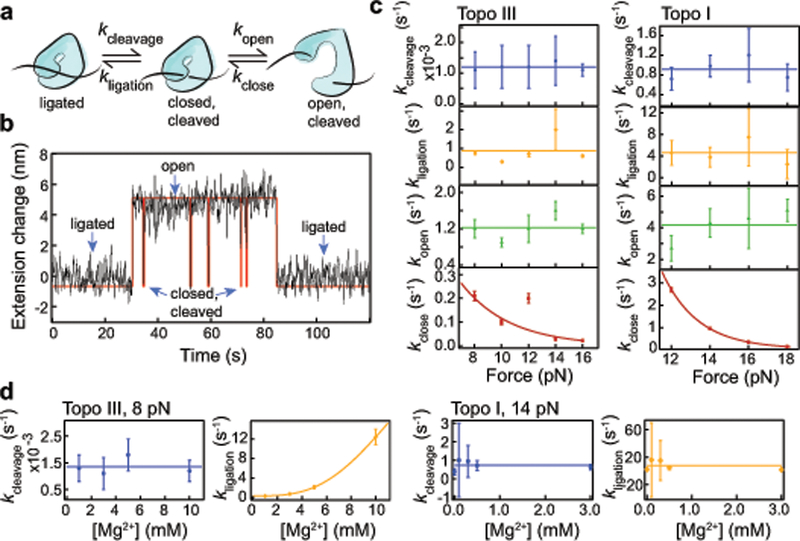
Three-state kinetics of topoisomerase IA gate dynamics. (a) Three-state kinetic model. (b) Example of a single kinetic cycle of topo III from cleavage to transient opening and closing of the gate, followed by religation. Black line is smoothed data, red line is the HMM fit from vbFRET. (c) Force dependent kinetic analysis of kcleavage (blue), kligation (orange), kopen (green), and kclose (red), ntethers = 8 for topo III, ntethers = 7 for topo I. Values were calculated from exponential fits of the lifetimes of each state (Supplementary Fig. 3–4). Error bars represent standard deviations of the fit coefficients. For kcleavage, kligation, and kopen, lines represent average values. kclose values were fit with k = k0exp(−FΔx/kBT). The fit returned Δx and k0 values of 1.10 ± 0.04 nm and 1.8 ± 0.3 s−1 respectively, for topo III and 2.2 ± 0.1 nm and 1642 ± 549 s−1 for topo I. (d) Magnesium concentration dependence of ligation and cleavage rates for topo III at 8 pN force (ntethers = 3, 8, 3, and 2 for 1, 3, 5, and 10 mM Mg2+, respectively) and topo I at 12 pN force (ntethers = 6, 4, 3, 5, and 2 for and 0, 0.1, 0.3, 0.5 and 3 mM Mg2+). The topo III magnesium-dependent ligation rate was fit with a sigmoid with a Km of 8.3 ± 1.2 mM Mg2+ and a Vmax of 17 ± 5 s−1.
