Figure 1. ER stressed astrocytes secrete a molecule(s) which upregulate ER stress responses in astrocytes and neurons.
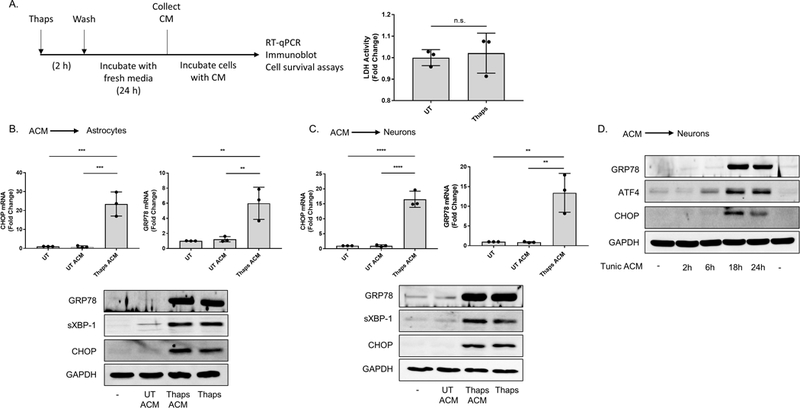
(A) Outline for the astrocyte conditioned media (ACM) experiments. Following treatment with the vehicle (DMSO) or 1.0 μM thapsigargin (Thaps) for 2 h, primary murine astrocyte cultures were washed with PBS three times, then incubated in fresh media for 24 h. After this period, the media (ACM) was collected. Lactate dehydrogenase (LDH) activity in the collected ACM was measured using the LDH assay. N = 3, p = 0.7 determined by Mann Whitney test. Not significant (n.s.) (B-C) Primary murine astrocytes (B) and murine HT-22 hippocampal neurons (C) incubated with control (UT ACM) or ER stressed ACM (Thaps ACM) for 6 h. CHOP and GRP78 mRNA expression was quantified using RT-qPCR. Immunoblot analysis was used to examine GRP78, spliced XBP-1 (sXBP-1) and CHOP protein expression. 1.0 μM (B) or 0.1 μM (C) Thaps was used as the positive control for B and C, respectively. GAPDH was used as the loading control. N = 3 independent cell culture preparations, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001 determined by one-way ANOVA with Tukey’s multiple comparisons test. (D) HT-22 neurons incubated with control or ER stressed ACM from tunicamycin (Tunic)-treated primary murine astrocytes for the indicated time points. Immunoblot analysis was used to examine GRP78, ATF4 and CHOP protein expression. GAPDH was used as the loading control.
