Figure 1.
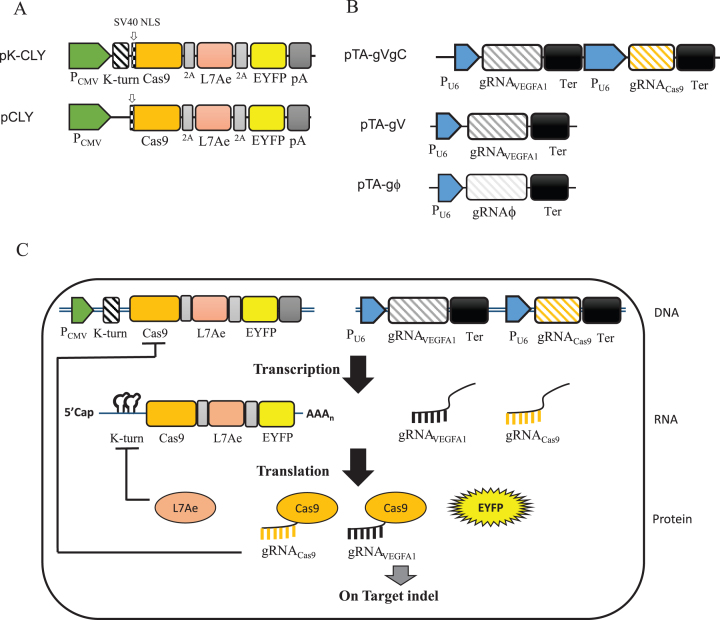
Design of the synthetic switch to restrict Cas9 transcription and translation. (A) Plasmids to co-express Cas9, L7Ae and EYFP, with (pK-CLY) or without (pCLY) two repeats of K-turn at the 5′ end. The three genes were linked in tandem by P2A sequences and driven by CMV promoter/enhancer. Cas9 was fused with an SV40 nuclear localization signal (NLS). pA, poly A signal. (B) Plasmids to express the gRNA. pTA-gVgC co-expressed two different gRNAs: gRNAVEGFA1 targeting human VEGFA1 gene on human chromosome 6 and gRNACas9 targeting the plasmid-borne Cas9 gene. pTA-gV expressed gRNAVEGFA1 while pTA-gϕ expressed a scramble gRNA (gRNAϕ) that targets no sequence on the chromosomes or plasmids. All gRNAs were driven by human U6 promoter. Ter, transcription termination sequence. (C) Schematic illustration of how the synthetic switch self-regulates Cas9 expression in the transcription and translation steps. Co-transfection of pK-CLY and pTA-gVgC into cells would lead to co-transcription of Cas9, L7Ae, EYFP, gRNAVEGFA1 and gRNACas9. Cas9 is translated and orchestrates with gRNAVEGFA1 to induce on-target indel mutation at VEGFA1 site while also associating with gRNACas9 to inhibit Cas9 transcription by cleaving the plasmid-borne Cas9 gene. Additionally, L7Ae is translated and in turn binds the K-turn motifs at the 5′ UTR of mRNA to suppress mRNA translation.