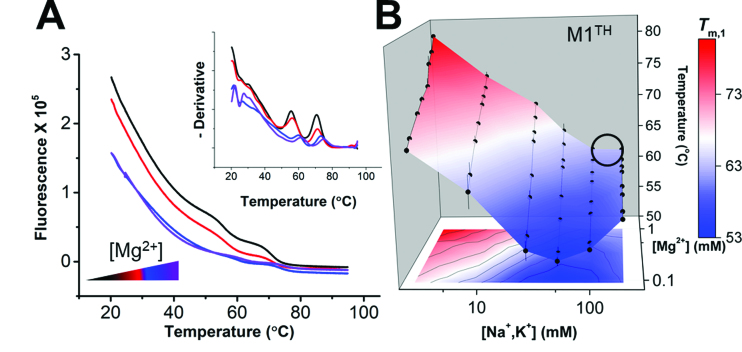
Figure 3.
Stability landscape of M1TH determined by differential scanning fluorimetry. (A) Melting profiles of M1TH monitored by RiboGreen fluorescence using DSF under four different magnesium concentrations (0.1, 0.2, 0.3 and 0.6 mM MgCl2, with magnesium concentrations increased from black to purple) in 51 mM total monovalent salt (equimolar NaCl and KCl). Inset: The negative derivative of the raw melting profile is used to determine the melting temperature transitions. Two melting transitions are recorded by DSF, with Tm values consistent with UV melt experiments (Table S2). (B) Tertiary thermal stability landscape of M1TH monitored by DSF under multiplexed ionic conditions (2.6, 5.7, 8.8, 15.1, 27.6, 52.6, 102.6 and 202.6 mM total monovalent salt; and 0.1, 0.2, 0.3, 0.5, 0.6, 0.8 and 1 mM MgCl2). n = 3 experiments; S.D. Thermostability of the unimolecular M1TH is considerably higher than physiological temperatures. A circle on the stability landscape indicates approximate physiological ionic conditions (∼150 mM monovalent, 1 mM MgCl2).