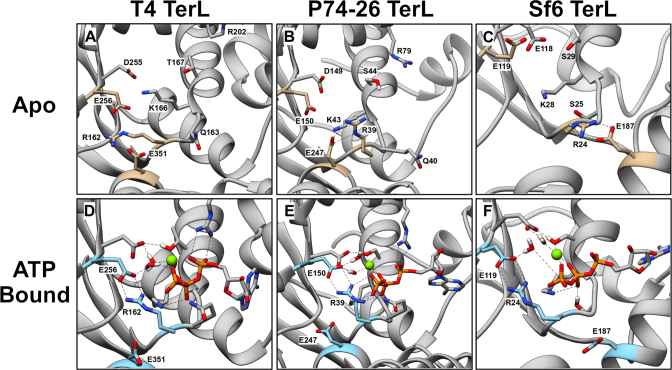
Figure 5.
Conformations of the ATP-Binding Pocket in WT TerL obtained from MD Simulations. Representative conformations of the apo binding pocket of TerLT4 (A), TerLP74-26 (B) and TerLSf6 (C). Walker motif residues and lid subdomain glutamate (see Table 1) are labeled and depicted as sticks. The WA arginine, catalytic glutamate and lid subdomain glutamate are highlighted in tan. Representative conformations of the ATP-bound binding pocket of TerLT4 (D), TerLP74-26 (E) and TerLSf6 (F). ATP is depicted as sticks, and Mg2+ is a green sphere. The WA arginine, catalytic glutamate and lid subdomain glutamate are labeled and are highlighted in cyan. In all three terminases, the conserved WA arginine (R162, R39, and R24 in TerLT4, TerLP74-26, and TerLSf6, respectively) interacts with the catalytic glutamate (E256, E150, E119 in TerLT4, TerLP74-26, and TerLSf6) near the γ-phosphate of ATP.