Figure 2.
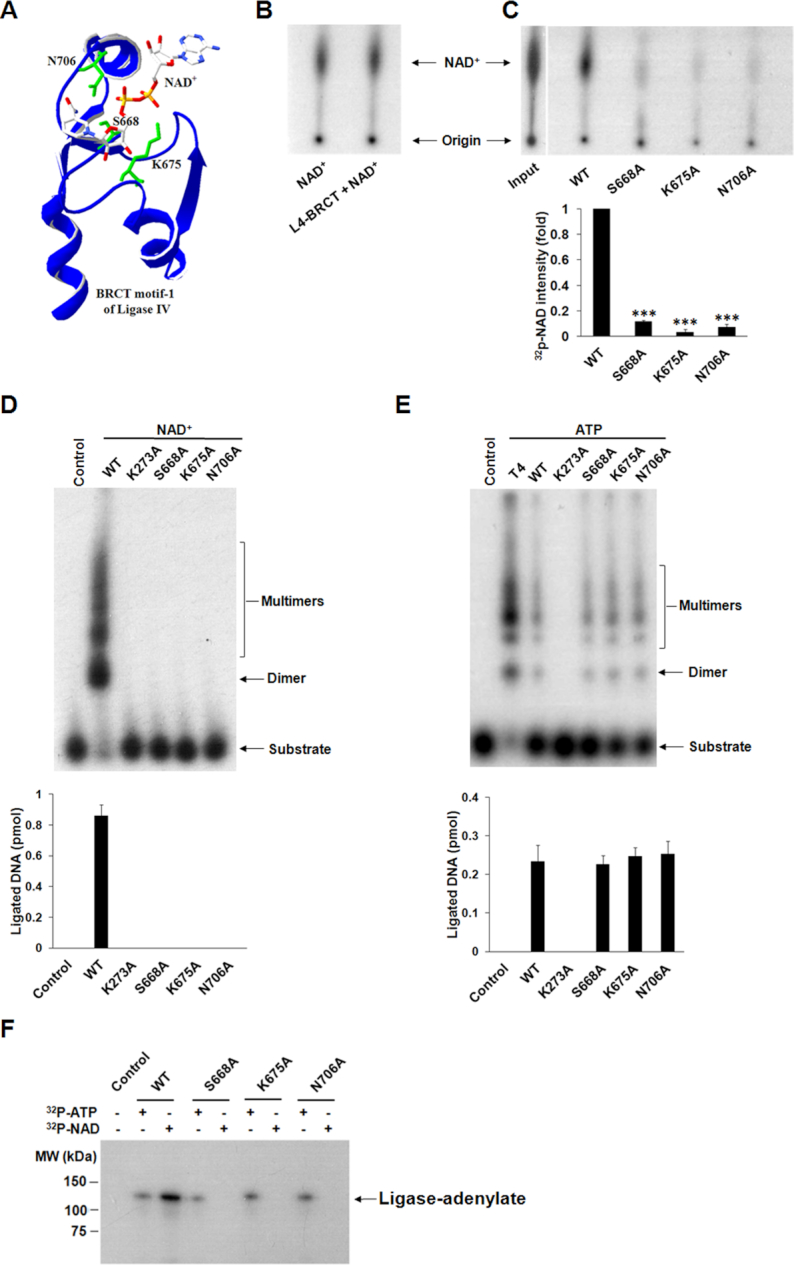
The BRCT domain is required for the NAD+-mediated ligation. (A) The binding mode of NAD+ in Ligase IV BRCT1 is predicted by GEMDOCK and the structure of the BRCT domain of Ligase IV (PDB: 2E2W). Three important residues in the BRCT domain of Ligase IV for the interactions with NAD+ are shown in green. (B and C) The wild-type BRCT domain interacts with NAD+. One micromolar wild-type (WT) BRCT domain or three variants were incubated with 500 nM NAD+ (10 nM 32P-NAD+ and 490 nM unlabeled NAD+) in the pull-down assays with 100 μl volume. The samples were analyzed by TLC. Relative 32P-NAD+ intensity compared to WT was presented as mean ± SD from three independent experiments. *** denotes P < 0.001. (D and E) The impact of the BRCT domain for the NAD+- and ATP-mediated ligation. The enzymatic activities of the three variants of Ligase IV were examined in the ligation assays with 4 nt-overhang DNA. Control means no protein. The K273A is a catalytic dead form of Ligase IV and was used as a negative control. Bar graphs show the mean of ligated products ± SD from three independent experiments. (F) Mutations in the BRCT domain abolish NAD+-mediated adenylation, but not ATP-mediated adenylation. Ligase IV wild-type (WT) and three mutants (25 nM) were incubated with 1.5 μM ATP or NAD+ (1% α-32P-ATP or 32P-NAD+) in 10 μl reaction buffer. The ligase-adenylate complexes were analyzed by 7.5% SDS-PAGE and autoradiography.
