Fig. 1.
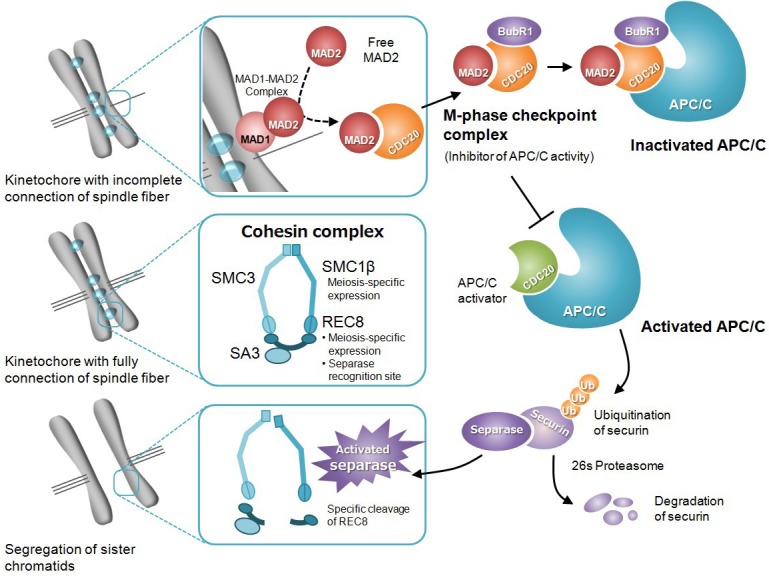
Summary of the molecular mechanism of spindle assembly checkpoint. MAD2, which binds with MAD1 on the kinetochore, monitors the connection between a spindle fiber and kinetochore. The MAD1-MAD2 complex serves as a catalyst to promote binding between a free MAD2 and CDC20. The MAD2-CDC20 complex forms a M-phase checkpoint complex (MCC) with other functional proteins, and suppresses the activity of APC/C. A fully connected spindle fiber on the kinetochore interrupts the signal from the spindle checkpoint. CDC20, released from MCC, behaves as an APC/C activator by binding with APC/C. Activated APC/C promotes ubiquitination of securin, which regulates separase activity. The separase activated by securin degradation cleaves REC8, which is contained in the cohesin complex. Finally, sister chromatids lose cohesin, an adhesion factor, and are segregated by tension from the spindle fiber.
