Abstract
Complex karyotype (CK) is a poor prognosis factor in hematological malignancies. Studies have shown that the presence of CK in myelodysplastic syndrome (MDS) can be associated with MDS progression to acute myeloid leukemia. The goal of this review was to examine the relationship between different types of CK with MDS, as well as its possible role in the deterioration and progression of MDS to leukemia. The content used in this paper has been obtained by a PubMed and Google Scholar search of English language papers (1975-2018) using the terms complex karyotype and myelodysplastic syndromes. A single independent abnormality can be associated with a good prognosis. However, the coexistence of a series of abnormalities can lead to CK, which is associated with the deterioration of MDS and its progression to leukemia. Therefore, CK may be referred to as a prognostic factor in MDS. The detection of independent cytogenetic disorders that altogether can result in CK could be used as a prognostic model for laboratory and clinical use.
Key words: Myelodysplastic syndrome, diagnosis, complex karyotype
Introduction
Complex karyotype (CK) is a classification of cytogenetic risks of hematologic malignancies associated with a poor prognosis,1 which has important applications in the diagnosis and prognosis of patients with hematological malignancies, including myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), B-cell lymphoblastic leukemia/lymphoma (B-ALL), chronic myeloid leukemia (CML), chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL), and plasma cell myeloma (PCM).1-3 There is no clear definition of CK for various types of hematologic malignancies. ≥3 independent cytogenetic abnormalities in most studies is considered as CK, but in Medical Research Council Acute Myeloid Leukemia 10 trial (MRC AML10), ≥5 independent cytogenetic abnormalities are deemed as CK.4 Haase et al. demonstrated that the increase in chromosomal abnormalities can be associated with reduced survival rates of MDS patients.5 In this respect, there are a number of complementary techniques to investigate important chromosomal events involved in the progression of treatmentrelated secondary myelodysplastic syndrome (t-MDS).6 For example, spectral karyotyping (SKY), a laboratory technique based on hybridization of 24 fluorescently labeled chromosome painting probes, as well as fluorescence in situ hybridization (FISH), and conventional cytogenetics are techniques used for analysis of CK and t-MDS.6,7
Complex karyotype as a prognosis predictor in myelodysplastic syndrome
MDS is an abnormality of bone marrow (BM) stem cells associated with monocytopenia, bicytopenies, or pancytopenia.5,8 Recent studies indicate that up to 30% of de novo MDS cases are associated with CK in initial cytogenetic evaluation.9 On the other hand, studies have indicated more frequent chromosomal abnormalities in t-MDS than de novo MDS. -7 (14 %), -5q (28%), -5 (11%), der (21q), +8, -7q, der (12q), t (1;7), -12, der (17q), der (3q), der (3q), and -18 are among the most common chromosomal abnormalities in t-MDS.10 International Prognostic Scoring System (IPSS) classifies MDS cytogenetic results into four groups of low, int-1, int-2, and high according to the risk of death or transformation to AML. Sconocchia et al. state that chromosome 7 anomalies and complex karyotype are placed on a high-risk class.11 MDS is often transformed to AML, but it can also transform to other leukemia, including acute lymphocytic leukemia (ALL).12 Each chromosomal abnormality in MDS is shown to be associated with a different prognosis; for example, del (5q), del (7q)/-5/-7, and +8 are considered to be associated with favorable, unfavorable, and indeterminate prognosis, respectively.13 On the other hand, has been observed that the higher numbers of the independent cytogenetic abnormalities in MDS patients (usually ≤3 independent cytogenetic abnormalities), the poorer prognosis of the MDS.14 As a result, assessment of CK in MDS can be useful in determining prognosis and finding new therapies (Table 1).
Table 1.
Chromosomal abnormalities associated with complex karyotype.
| Cytogenetics | Mutations | Clinical findings | Consequence | Prognosis | Ref. |
|---|---|---|---|---|---|
| -5, -7, -17 | Mutation in chromatin modifiers like TET2,DNMT3A, ASXL1 | ➤ The simultaneous occurrence of TP53 mutations with these mutations and cytogenetics can lead to CK ➤ The gene encoding TP53 is located on 17p, so the removal of chromosome 17 can cause non-stop cell cycle at G1 stage, inability to induce apoptosis, which ultimately lead to cancer progression due to inability to induce the expression of p21WAF1/CIPl/Sdil and inhibitor of CDK2, 3, 4 and 6. |
TP53 mutations can lead to additive cell growth and also increase cell survival, proliferation, evasion of apoptosis, and chemoresistance | Poor | 15,19,20,63 |
| -6, add (8) (q24), der (10) t (10;11), (q28, q13), -11, add (12) (p13), + 13, +13, add (17) (p13), -18, -21, -22, + 1-3 mar, 0 ~ 2dmi [ Cp20], del (11) (q21q23), add (17) (p13), 20.0 ~ 3dmin [16] / 46 | -17p/TP53 mutation and mutation in amplified genes like C-MYC, MYCN, MDM2 and EIF5A2 | ➤ The simultaneous occurrence of DMS* with these mutations and cytogenetics can lead to CK. ➤ DMS can be treated with low-dose HU and ionizing radiation. ➤ MDM2 is a MDM2 gene protein product identified as an amplified gene on a murine double-minute chromosome. MDM2 can act as an ubiquitin-protein ligase to ubiquitinated p53 and induce p53 nuclear export and its degradation in cytoplasmic proteasomes by binding P53 with high affinity. On the other hand, MDMX can act as an upregulator for MDM2. |
MDM2 and MDMX* mutations can lead to inhibition of apoptosis, increased cell proliferation, and cancer progression | Poor | 30,31,32,34, 35, 64 |
| Der (9p), der(12) dic (12;?19), +15, −18, –16, del(5) (q13) | There is still no precise information on mutations that occur predominantly with RC | ➤ The simultaneous occurrence of RC with this cytogenetics can lead to CK. ➤ RC12 contains MDM2, SAS, CDK4, and HMGIC genes that increase proliferation, and expression of these genes can be associated with an increase in carcinogenesis. |
RC is associated with decreased cell apoptosis and increased proliferation in most types of human neoplasia. | Poor | 38,39,65 |
| Der (1) t(1:2), -7, 15, -18, -19 | Deletion of some genes like RPS14, EGR1, NPM1, APC, CTNNA1 and TP53 mutation | ➤ The simultaneous occurrence of the del (5q) with these mutations and cytogenetics can lead to CK. ➤ Del(5q) can be treated with lenalidomide. ➤ CSNK1A1 gene is located on chromosome 5, and studies have shown that heterozygous loss of CSNK1A1 is well-tolerated and may led to the clonal advantage of cells. Nevertheless, homozygous loss of CSNK1A1 can lead to cell cycle arrest and apoptosis due to p53 activation. |
MDS with del(5q) is associated with normal to increased megakaryocytes with hypo-lobulated nuclei, erythroid hypoplasia, and normal or increased platelet count | Poor with AML progression | 22,24,25 |
| -5q,-7q-12, -17, -5t (9;19) (p13; q13), t (6;12) (p21; q13), der (7) t (7;20) (p13;?),t (7;18) (q11 ;q11), der (18) t(18;22) (q21;q?), der (19) t (7;19) (?;?) | mutation in 11q23 | ➤ The simultaneous occurrence of der (11) t (11;12) (q23; q13) with these mutations and cytogenetics can lead to CK. ➤ Chromosomal translocations involving 11q23 can led to rearrangements in MLL gene. ➤ Therefore, the protein encoded by this gene can be involved in abnormal control of proliferation and differentiation in monocytic progenitor cells and transform hematopoietic cells into leukemia stem cells. |
der (11) t(11;12) (q23;q13) is associated with pathogenesis of advanced-stage MDS and increased cell proliferation | Poor | 41-45,66 |
| +11, +22, -20, add (17) (q25), del(5)(q33) | Isochromosome of the long arm of chromosome 17q and 18 | ➤ Myelofibrosis is mainly associated with these mutations and cytogenetics that contribute to the development of CK. ➤ BCL-2 gene is localized on 18q21. BCL-2 gene product is a protein with anti-apoptotic property. Studies show that isochromosome 18q may mildly increase myeloblasts in peripheral blood through a gene dosage effect. ➤ Isochromosome 18q and myelofibrosis are considered as two markers for poor prognosis |
Isochromosome 18q may be associated with decreased cell apoptosis, increased myeloblasts in blood and eventually, worsening of MDS presenting with myelofibrosis as two markers for poor prognosis | Poor | 47-49 |
| T(4;11 ((q21;q23), del (5) (q13q33),t (12;13) (p13;q21) [31] / 92, i (17) (q10) [2] /46,XX [2] | mutation in RAS proteins like Point mutation N-RAS at codon 12 or 13 | ➤ The simultaneous occurrence of N-RAS Point mutation with this cytogenetics can lead to CK. ➤ RAS proteins are involved in control of cell proliferation, differentiation, and survival through the two pathways of MAPK and PI3K. Studies show that in MAPK pathway, mutations in RAS proteins may result in inability of RAS-GAP protein to activate the inherent GTase enzyme. As a result, due to lack of hydrolysis of GTP molecules, RAS remains active as an alternative for GTP molecules, so this mechanism can cause increasing pro tumorigenic effects by amplifying signaling in the MAPK pathway. On the other hand, in PI3K pathway, it is hypothesized that mutations in RAS, can lead to increased PI3K activity and finally increase cellular growth and evasion of apoptosis through induced mutations in PIK3CA and encoding a truncated version of P110α. |
Point mutation N-RAS is associated with disorder in control of cell proliferation, differentiation, survival, and eventual enhancement of cancer progression | Poor | 53,60-62,67, 68 |
*MDMX (MDM2 and MDM4, HDM2, and HDMX in humans). CK: complex Karyotype, Del: deletion, MDS: myelodysplastic syndrome, DMS: double minute chromosomes, RC: ring chromosome, SCE: sister chromatid exchange, t-MDS: therapy-related secondary myelodysplastic syndrome, 17p: short arm of 17 chromosomes, CDK: Cyclin dependent kinases, MDM: mouse double minute, MLL; myeloid /lymphoid leukemia or mixed lineage leukemia, MAPK: mitogen-activated protein kinases, PI3K: phosphoinositide-3 kinase.
TP53 mutations and del (5q) with complex karyotype
TP53 mutations
TP53 is a tumor suppressor gene (TSGs), the mutation of which can be associated with increased proliferation and survival of tumor cells.15 In this regard, Wang et al. in their recent study have shown that most MDS patients harbor TP53 mutation and therefore have a poor prognosis.16 Moreover, del (5q) /-5 > del (7q) /-7 <del (17p) /-17 is observed in MDS patients with CK. No relationship has been found between del (7q) /-7 and del (5q) /-5 with TP53;16-18 on the other hand, since TP53 gene is located on 17p, coexistence of mutation in 17p region and deletion of chromosome 17 can involve both TP53 alleles and result in deactivation of both copies of TP53 gene in a majority of cases. Therefore, simultaneous occurrence of mutation and del )17p(, which is associated with a poorer prognosis than the time they occur separately, leads to increased carcinogenesis.15,19 Also, Cazzola et al. demonstrated that coexistence of mutation in chromatin modifiers (e.g., TET2, DNMT3A, and ASXL1) and TP53 mutation leads to MDS progression and a poorer prognosis (Table 1).20 Therefore, it may be possible to predict MDS prognosis/progression and transformation to different types of leukemia by examining TP53 mutations and CK in MDS/progressive MDS to leukemia.
Del (5q)
Del (5q) involves the deletion of q31-q33 on the long arm of chromosome 521 that is observed in 10-15% of MDS patients.22 In this regard, it has been observed that deletion in 5q32-33 region is the most common deletion in del(5q). On the other hand, deletion in 5q31 region is accompanied by a higher risk of MDS and AML.22 Coexistence of del (5q) and a set of chromosomal abnormalities such as der (1) t(1:2), -7, -15, -18, -19 with TP53 mutation can lead to CK.23,24 Ammatuna et al. demonstrated that MDS with single chromosomal abnormality of del (5q) has a more favorable prognosis in comparison to other chromosomal abnormalities, while del (5q) together with CK will have an unfavorable prognosis.25 Also, since 5q region harbors genes such as CSNK1A1, RPS14, EGR1, miR-145 and miR-146a, it can be stated that the absence of these genes in del (5q) can lead to selective sensitivity to lenalidomide.22 On the other hand, it has been observed that CK (including at least two abnormalities in addition to del 5q) in MDS patients is associated with a higher risk of AML progression that can affect the response to lenalidomide. For example, TP53 mutation can lead to reduced sensitivity to lenalidomide, as well as selective growth of mutant clones.22,24 Studies have shown that low risk MDS with isolated del (5q) responds well to lenalidomide treatment, but high risk MDS, which is usually associated with CK, has a poor response to lenalidomide treatment.11,24,26 However, Lionel Ades et al. stated that a combination of chemotherapy stimulants like classical Daunorubicin-AraC with lenalidomide can entail a better response.27 Therefore, more studies with a higher number of patients are required to assess the effect of drugs such as lenalidomide on the improvement of MDS patients with del (5q) chromosomal abnormality. In this regard, it has been observed that patients with an isolated del (5q) have a better prognosis than those with other chromosomal abnormalities; however, if this chromosomal deletion is accompanied by mutations in TP53, there will be a much poorer prognosis.26 There are a number of genes on 5q chromosome, including RPS14, EGR1, NPM1, APC, and CTNNA1, each of which is related to TP53 in some way or other. For example, NPM1 gene modulates the activity of TP53 and CDKN2A tumor suppressors, and both EGR1 (a direct transcriptional regulator of tumor suppressor genes like TP53, CDKN1A, and PTEN) and APC genes are involved in encoding tumor suppressor proteins (TSP) (Table 1).27-29 Therefore, further studies on the relationship between TP53 mutation and del (5q) are warranted.
Double minute chromosomes and complex karyotype
Double-minute chromosomes (DMs) are replicated DNA sequences in tumor cells that contain amplified genes such as CMYC, MYCN, MDM2, and EIF5A2,30-32 which are typically involved in increased carcinogenesis and poor prognosis of cancer. Bao et al. demonstrated that low-dose hydroxyurea (HU) treatment could reduce the frequency of DMs in human cancer cells. On the other hand, ionizing radiation can eliminate amplified genes on DMs;33 therefore, it can be stated that HU and ionizing agents play a role in the improvement of cancer prognosis. On the other hand, it has been shown that DMs are associated with the frequency of sister chromatid exchange (SCE) in cancer cells and the increased malignancy in cancer.34 In a study conducted on patients with myeloid malignancies and MDS, it was observed that all patients with MLL gene amplification on their DMs were associated with CK like -6, add (8) (q24), der (10) t(10;11), (q28, q13), -11, add (12) (p13), + 13, +13, add (17) (p13), -18, -21, -22, + 1-3 mar, 0~2dmin [Cp20], del (11) (q21q23), add (17) (p13), 20.0 ~ 3dmin [16] / 46, idem, + mar [4].35 On the other hand, patients with DMs and CK were associated with 17p/TP53 deletion, so it can be stated that there is a relationship between DMs and CK with a poor prognosis (Table 1).35 Consequently, conducting further studies on the methods reducing/inhibiting DMs in cancer cells might prevent poor prognosis or MDS progression.
Ring chromosome and complex karyotype
Ring chromosome (RC) is a circular DNA molecule36 formed by merging of two broken ends of a chromosome, a broken end with the end of telomeres of a chromosome, or the two ends of telomeres of a chromosome.37 RC is a rare disorder; However, in 75% of AML patients and 97% of MDS patients holder RC mutation, the CK [such as der (9p), der (12) dic (12 ;?19), +15, - 18] have been observed.38,39 Research has shown that RC increase in MDS is associated with progression of MDS to AML. Moreover, MDS patients with CK+RC+ have shorter leukemiafree survival than those with RC+CK-. On the other hand, CK+RC+ AML patients have an overall survival (OS) similar to CK+RC- AML patients, but they have a shorter OS compared to CK-RC+ patients (Table 1).39,40
Der (11) t (11;12) (q23; q13) and complex karyotype
Der (11) t (11;12) (q23; q13) is an unbalanced translocation (partial monosomy or trisomy) that occurs non-randomly and may be associated with the pathogenesis of advanced stage MDS.41,42 Investigations show that der (11) t(11;12)(q23;q13) in refractory anemia with excess of blasts in transformation (RAEB-t), which is a type of MDS, can be associated with a series of chromosomal aberrations such as -12, -17, -5, t(9;19) (p13;q13), t(6;12) (p21;q13),43 der(7) t(7;20) (p13;?) t(7;18) (q11;q11), der(19) t(7;19), der(18) t(18;22) (q21;q?), deletions of 5q and 7q and lead to CK. CK mainly occurs in advanced stage MDS and is associated with a poor prognosis (Table 1).41,42,44,45 Therefore, by conducting further studies on the relationship between der (11) t(11;12) (q23;q13) and advanced stage MDS, der (11) t (11;12) (q23;q13) might prove a target for advanced stage MDS therapeutic methods.
Myelofibrosis and complex karyotype
Myelofibrosis is a rare disorder associated with BM fibrosis and accumulation of immature precursors of three hematopoietic cell lines.46-47 It can be associated with MDS and a number of chromosomal abnormalities such as -18, -20, +22, +11, add (17) (q25), del(5)(q33), and isochromosome of long arm of chromosome 18.47-49 Elizabeth E et al. indicated that if MDS is accompanied with fibrosis and chromosomal abnormalities, the prognosis will be poorer than the case MDS is associated with chromosomal abnormalities without fibrosis (Table 1).50 Therefore, BM biopsy together with cytogenetic study is suggested in MDS patients presenting with a decrease in hematopoietic cell lineages and splenomegaly.
N-RAS point mutation and complex karyotype
RAS isoforms, which include N-, K-, and H-RAS, are GTPases that play a role in cell growth, differentiation, migration, and apoptosis.51,52 Therefore, mutations in RAS isoforms are dominant mutations in MDS and AML subtypes such as acute megakaryoblastic leukemia (AML-M7).53 Studies have shown that a point mutation in the gene encoding N-RAS at codon 12 is one of the most common mutations in MDS and AML-M7, which could possibly contribute to the progression of MDS to AML-M7.54,55 A number of studies have suggested a mechanism for MDS development via uncontrolled proliferation of hematopoietic cells independent of growth factors, which is followed by mutations in RAS proto-oncogene and its signaling pathway.56-58 Nevertheless, other studies indicate that compared to other mutations in RAS isoforms, aspartate substitution with glycine in N-RAS codon 12 is capable of maintaining ≥40% activity of wild type GTPases, which can be considered as another mechanism for mutation in the cell.55,59 As a result, no exact mechanism has been described for the relationship between mutation of RAS isoforms and MDS progression to AML. In this regard, during the progression of MDS to AML, N-RAS point mutation can occur together with chromosomal anomalies such as t (4;11) (q21;q23), del(5) (q13q33), t (12;13) (p13;q21) [31] /92, XXXX, idem × 2 [6] /46, XX, i (17) (q10) [2] /46, XX [2] to result in CK;60-62 therefore, these chromosomal abnormalities can be associated with a poor prognosis. Teresa de Souza Fernandez et al. indicated that isochromosome 17q and polyploidy were probably caused by genetic instability due to transformation of MDS into malignant leukemia (Table 1).62-65
Figures 1 and 2 show several examples of independent chromosome aberrations in CK.
Figure 1.
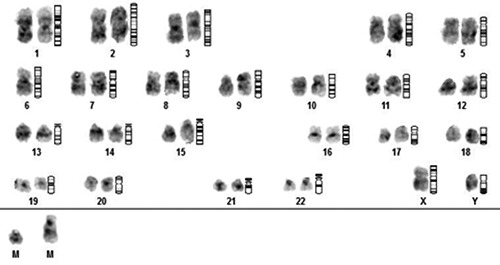
Examples of CK, including independent chromosome aberrations such as -6, der (9) (12), +2mar.
Discussion and Conclusions
CK, which refers to independent chromosomal abnormalities (≥3-5 independent chromosomal abnormalities), is among the worst risk factors of MDS patients. An increase in the number of chromosomal abnormalities in MDS has been related with poorer prognosis, decreased OS, and increased progression to AML, although the prognosis of an isolated chromosomal abnormality can be different with its prognosis in CK. For example, del (5q) by itself is associated with a good prognosis and lower progression rates to AML, while when associated with other genetic abnormalities to form CK, del (5q) has a poorer prognosis and higher AML progression rates. On the other hand, patients with CK having at least four independent chromosomal abnormalities show an increased risk of relapse. CK can also affect a number of drugs applied to treat MDS or leukemia, reducing the effectiveness of the drug in treatment of the disease. However, according to studies, it may be argued that the degree of CK interference with effectiveness of drug can be different based on the types of independent chromosomal abnormalities that form CK. Therefore, by conducting studies focusing on a series of chromosomal abnormalities involved in the formation of CK, approaches can be introduced to determine the prognosis of MDS and prevent the progression of MDS to leukemia and its relapse.
Figure 2.
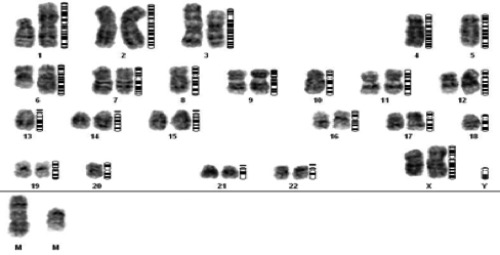
Examples of CK, involving independent chromosome aberrations such as -4, -5, -8, -10, -13, -18, -20, der (1)(6), +2mar.
Acknowledgments
We wish to thank all our colleagues at Noorgene Genetic and Clinical Laboratory, Ahvaz, Iran.
References
- 1.Peterson JF. The complexities of defining a complex karyotype in hematological malignancies: a need for standardization? Acta Haematologica 2017;138:65-6. [DOI] [PubMed] [Google Scholar]
- 2.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391-405. [DOI] [PubMed] [Google Scholar]
- 4.Gohring G, Michalova K, Beverloo HB, et al. Complex karyotype newly defined: the strongest prognostic factor in advanced childhood myelodysplastic syndrome. Blood 2010;116:3766-9. [DOI] [PubMed] [Google Scholar]
- 5.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 2007;110:4385-95. [DOI] [PubMed] [Google Scholar]
- 6.Cohen N, Trakhtenbrot L, Yukla M, et al. SKY detection of chromosome rearrangements in two cases of tMDS with a complex karyotype. Cancer Genet Cytogenet 2002;138:128-32. [DOI] [PubMed] [Google Scholar]
- 7.Schrock E, du Manoir S, Veldman T, et al. Multicolor spectral karyotyping of human chromosomes. Science (New York, NY) 1996;273:494-7. [DOI] [PubMed] [Google Scholar]
- 8.Cogle CR, Saki N, Khodadi E, et al. Bone marrow niche in the myelodysplastic syndromes. Leuk Res 2015;39:1020-7. [DOI] [PubMed] [Google Scholar]
- 9.Trost D, Hildebrandt B, Beier M, et al. Molecular cytogenetic profiling of complex karyotypes in primary myelodysplastic syndromes and acute myeloid leukemia. Cancer Genet Cytogenet 2006;165:51-63. [DOI] [PubMed] [Google Scholar]
- 10.Heim S, Mitelman F. Cancer cytogenetics: chromosomal and molecular genetic aberrations of tumor cells. London: John Wiley & Sons; 2015. [Google Scholar]
- 11.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007;25:3503-10. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Nakazato T, Kizaki M, et al. Transformation of myelodysplastic syndrome to acute lymphoblastic leukemia: a case report and review of the literature. Int J Hematol 2004;79:147-51. [DOI] [PubMed] [Google Scholar]
- 13.Musilova J, Michalova K, Zemanova Z, et al. Karyotype at diagnosis, subsequent leukemic transformation and survival in myelodysplastic syndrome. Czechoslovak MDS Cooperative Group; 1995. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012;120:2454-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivlin N, Brosh R, Oren M, et al. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes & Cancer 2011;2:466-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Routbort MJ, Tang Z, et al. Characterization of TP 53 mutations in low-grade myelodysplastic syndromes and myelodysplastic syndromes with a non-complex karyotype. Eur J Haematol 2017;99:536-43. [DOI] [PubMed] [Google Scholar]
- 17.Kulasekararaj AG, Smith AE, Mian SA, et al. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol 2013;160:660-72. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen-Bjergaard J, Andersen MK, Christiansen DH, et al. Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia. Blood 2002;99:1909-12. [DOI] [PubMed] [Google Scholar]
- 19.Stengel A, Kern W, Haferlach T, et al. The impact of TP53 mutations and TP53 deletions on survival varies between AML, ALL, MDS and CLL: an analysis of 3307 cases. Leukemia 2017;31:705-11. [DOI] [PubMed] [Google Scholar]
- 20.Cazzola M, Della Porta MG, Malcovati L. The genetic basis of myelodysplasia and its clinical relevance. Blood 2013;122:4021-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giagounidis AA, Germing U, Wainscoat JS, et al. The 5q-syndrome. Hematology (Amsterdam, Netherlands) 2004;9:271-7. [DOI] [PubMed] [Google Scholar]
- 22.Hosono N, Makishima H, Mahfouz R, et al. Recurrent genetic defects on chromosome 5q in myeloid neoplasms. Oncotarget 2017;8:6483-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giagounidis AAN, Haase S, Heinsch M, et al. Lenalidomide in the context of complex karyotype or interrupted treatment: case reviews of del(5q)MDS patients with unexpected responses. Ann Hematol 2007;86:133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.List A, Ebert BL, Fenaux P. A decade of progress in myelodysplastic syndrome with chromosome 5q deletion. Leukemia 2018;32:1493-9. [DOI] [PubMed] [Google Scholar]
- 25.Ammatuna E, Panetta P, Agirre X, et al. NPM1 gene deletions in myelodysplastic syndromes with 5q- and complex karyotype. Haematologica 2011;96:784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ades L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood 2009;113:3947-52. [DOI] [PubMed] [Google Scholar]
- 27.Ades L, Prebet T, Stamatoullas A, et al. Lenalidomide combined with intensive chemotherapy in acute myeloid leukemia and higher-risk myelodysplastic syndrome with 5q deletion. Results of a phase II study by the Groupe Francophone Des Myelodysplasies. Haematologica 2017;102:728-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron V, Adamson ED, Calogero A, et al. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther 2006;13:115-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollgard L, Saft L, Treppendahl MB, et al. Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica 2011;96:963-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alitalo K, Schwab M, Lin CC, et al. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci 1983;80:1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanDevanter DR, Piaskowski VD, Casper JT, et al. Ability of circular extrachromosomal DNA molecules to carry amplified MYCN proto-oncogenes in human neuroblastomas in vivo. J Natl Cancer Inst 1990;82:1815-21. [DOI] [PubMed] [Google Scholar]
- 32.Fakharzadeh SS, Rosenblum-Vos L, Murphy M, et al. Structure and organization of amplified DNA on double minutes containing the mdm2 oncogene. Genomics 1993;15:283-90. [DOI] [PubMed] [Google Scholar]
- 33.Bao Y, Liu J, You J, et al. Met promotes the formation of double minute chromosomes induced by Sei-1 in NIH-3T3 murine fibroblasts. Oncotarget 2016;7:56664-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Liu P, Meng X, et al. Association between sister chromatid exchange and double minute chromosomes in human tumor cells. Mol Cytogenet 2015;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huh YO, Tang G, Talwalkar SS, et al. Double minute chromosomes in acute myeloid leukemia, myelodysplastic syndromes, and chronic myelomonocytic leukemia are associated with micronuclei, MYC or MLL amplification, and complex karyotype. Cancer Genet 2016;209:313-20. [DOI] [PubMed] [Google Scholar]
- 36.Habib AGK, Sugiura K, Ueno M. Chromosome passenger complex is required for the survival of cells with ring chromosomes in fission yeast. PLoS One 2018;13:e0190523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilherme RS, Ayres Meloni VF, Kim CA, et al. Mechanisms of ring chromosome formation, ring instability and clinical consequences. BMC Med Genet 2011;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim T, Bershteyn M, Wynshaw-Boris A. Chromosome therapy. Correction of large chromosomal aberrations by inducing ring chromosomes in induced pluripotent stem cells (iPSCs). Nucleus (Austin, Tex) 2014;5:391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenbaum MW, Pozdnyakova O, Geyer JT, et al. Ring chromosome in myeloid neoplasms is associated with complex karyotype and disease progression. Human Pathol 2017;68:40-6. [DOI] [PubMed] [Google Scholar]
- 40.Ganguly B, Dolai T, De R, et al. Spectrum of complex chromosomal aberrations in a myelodysplastic syndrome and a brief review. J Cancer Res Ther 2016;12:1203-6. [DOI] [PubMed] [Google Scholar]
- 41.Fenaux P. Chromosome and molecular abnormalities in myelodysplastic syndromes. Int J Hematol 2001;73:429-37. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Hato A, Minagawa K, et al. Unbalanced translocation der (11) t (11; 12)(q23; q13): a new recurrent cytogenetic aberration in myelodysplastic syndrome with a complex karyotype. Cancer Genet Cytogenet 2004;155:67-73. [DOI] [PubMed] [Google Scholar]
- 43.Michels SD, Saumur J, Arthur DC, et al. Refractory anemia with excess of blasts in transformation hematologic and clinical study of 52 patients. Cancer 1989;64:2340-6. [DOI] [PubMed] [Google Scholar]
- 44.Fenaux P, Morel P, Lai J. Cytogenetics of myelodysplastic syndromes. Semin Hematol 1996;33:127-38. [PubMed] [Google Scholar]
- 45.Mecucci C. Molecular features of primary MDS with cytogenetic changes. Leukemia research 1998; 22: 293-302. [DOI] [PubMed] [Google Scholar]
- 46.Bearman RM, Pangalis GA, Rappaport H. Acute (“malignant”) myelosclerosis. Cancer 1979;43:279-93. [DOI] [PubMed] [Google Scholar]
- 47.Sultan C, Imbert M, Jouault H, Scoazec JY. Myelodysplastic syndromes. Acta Haetmatol 1987;1:91-3. [DOI] [PubMed] [Google Scholar]
- 48.Imbert M, Nguyen D, Sultan C. Myelodysplastic syndromes (MDS) and acute myeloid leukemias (AML) with myelofibrosis. Leuk Res 1992;16:51-4. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y, Cao Y, Gao Y, et al. An isochromosome of the long arm of chromosome 18 in a patient with myelodysplastic syndrome with myelofibrosis. Cancer Genet Cytogenet 1995;79:149-52. [DOI] [PubMed] [Google Scholar]
- 50.Allen EF, Lunde JH, McNally R, et al. A case of acute myelofibrosis with complex karyotypic changes: a type of myelodysplastic syndrome. Cancer Genet Cytogenet 1996;90:24-8. [DOI] [PubMed] [Google Scholar]
- 51.Cox AD, Der CJ. Ras history: the saga continues. Small GTPases 2010;1:2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertacchini J, Ketabchi N, Mediani L, et al. Inhibition of Rasmediated signaling pathways in CML stem cells. Cell Oncol (Dordrecht) 2015;38:407-18. [DOI] [PubMed] [Google Scholar]
- 53.Vasioukhin V, Anker P, Maurice P, et al. Point mutations of the N-ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br J Haematol 1994;86:774-9. [DOI] [PubMed] [Google Scholar]
- 54.de Souza Fernandez T, Menezes de Souza J, Macedo Silva ML, et al. Correlation of N-ras point mutations with specific chromosomal abnormalities in primary myelodysplastic syndrome. Leuk Res 1998;22:125-34. [DOI] [PubMed] [Google Scholar]
- 55.Nakagawa T, Saitoh S, Imoto S, et al. Multiple point mutation of N-ras and K-ras oncogenes in myelodysplastic syndrome and acute myelogenous leukemia. Oncol 1992;49:114-22. [DOI] [PubMed] [Google Scholar]
- 56.Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989;49:4682-9. [PubMed] [Google Scholar]
- 57.Downward J. Targeting RAS signalling pathways in cancer therapy. Nature Rev Cancer 2003;3:11-22. [DOI] [PubMed] [Google Scholar]
- 58.Badar T, Patel KP, Thompson PA, et al. Detectable FLT3-ITD or RAS mutation at the time of transformation from MDS to AML predicts for very poor outcomes. Leuk Res 2015;39:1367-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chin YM1, Bosco JJ, Koh CL. Analysis of ras gene mutations in acute myeloid leukemia by the polymerase chain reaction and oligonucleotide probes. Singapore Med J 1992;33(1):48-50. [PubMed] [Google Scholar]
- 60.Ohyashiki K, Yoshida MA, Ohyashiki J, et al. Involvement of chromosomes 4, 11, and 17 in a case of myelodysplastic syndrome. Cancer Genet Cytogenet 1985;18:265-73. [DOI] [PubMed] [Google Scholar]
- 61.Suciu S, Kuse R, Weh HJ, et al. Results of chromosome studies and their relation to morphology, course, and prognosis in 120 patients with de novo myelodysplastic syndrome. Cancer Genet Cytogenet 1990;44:15-26. [DOI] [PubMed] [Google Scholar]
- 62.de Souza Fernandez T, Ornellas MH, de Carvalho LO, et al. Complex karyotype and N-RAS point mutation in a case of acute megakaryoblastic leukemia (M7) following a myelodysplastic syndrome. Cancer Genet Cytogenet 2000;117:104-7. [DOI] [PubMed] [Google Scholar]
- 63.Shaw PH. The role of p53 in cell cycle regulation. Pathol Res Pract 1996;192:669-75. [DOI] [PubMed] [Google Scholar]
- 64.Neochoritis C, Estrada-Ortiz N, Khoury K, et al. Chapter twelve - p53-MDM2 and MDMX antagonists. Desai MC, eds. Annual reports in medicinal chemistry. Academic Press; 2014. pp 167-87. [Google Scholar]
- 65.Gisselsson D, Hoglund M, Mertens F, et al. The structure and dynamics of ring chromosomes in human neoplastic and nonneoplastic cells. Human Genet 1999;104:315-25. [DOI] [PubMed] [Google Scholar]
- 66.Munoz L, Nomdedeu JF, Villamor N, et al. Acute myeloid leukemia with MLL rearrangements: clinicobiological features, prognostic impact and value of flow cytometry in the detection of residual leukemic cells. Leukemia 2003;17:76-82. [DOI] [PubMed] [Google Scholar]
- 67.Zenonos K, Kyprianou K. RAS signaling pathways, mutations and their role in colorectal cancer. World J Gastrointest Oncol 2013;5:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fearon ER. Molecular genetics of colorectal cancer. Ann Rev Pathol 2011;6:479-507. [DOI] [PubMed] [Google Scholar]


