Abstract
Purpose
Bone tissue engineering is helpful in finding alternatives to overcome surgery limitations. Bone growth and repair are under the control of biochemical and mechanical signals; therefore, in recent years several approaches to improve bone regeneration have been evaluated. Osteo-inductive biomaterials, stem cells, specific growth factors and biophysical stimuli are among those. The aim of the present study was to evaluate if low-intensity pulsed ultrasound stimulation (LIPUS) treatment would improve the colonization of an MgHA/Coll hybrid composite scaffold by human mesenchymal stem cells (hMSCs) and their osteogenic differentiation. LIPUS stimulation was applied to hMSCs cultured on MgHA/Coll hybrid composite scaffold in osteogenic medium, mimicking the microenvironment of a bone fracture.
Methods
hMSCs were seeded on MgHA/Coll hybrid composite scaffold in an osteo-inductive medium and exposed to LIPUS treatment for 20 min/day for different experimental times (7 days, 14 days). The investigation was focused on (i) the improvement of hMSCs to colonize the MgHA/Coll hybrid composite scaffold by LIPUS, in terms of cell viability and ultrastructural analysis; (ii) the activation of MAPK/ERK, osteogenic (ALPL, COL1A1, BGLAP, SPP1) and angiogenetic (VEGF, IL8) pathways, through gene expression and protein release analysis, after LIPUS stimuli.
Results
LIPUS exposure improved MgHA/Coll hybrid composite scaffold colonization and induced in vitro osteogenic differentiation of hMSCs seeded on the scaffold.
Conclusions
This work shows that the combined use of new biomimetic osteo-inductive composite and LIPUS treatment could be a useful therapeutic approach in order to accelerate bone regeneration pathways.
Keywords: Human mesenchymal stem cells, Low intensity pulsed ultrasounds, Osteogenic differentiation, MgHA/Coll hybrid composite scaffold
Introduction
Bone tissue engineering uses both life sciences and engineering knowledge to regenerate or improve the function of injured bone tissue via several approaches: osteogenic biomaterials (1-2-3-4), stem cells (5-6-7-8-9) and supplementation with external specific growth factors and/or biophysical stimuli (10-11-12-13-14). Many preclinical and clinical studies were focused on the evaluation of the efficacy of these approaches, alone or in combination. Scaffolds play a key role in bone tissue engineering providing a 3-dimensional environment and a highly interconnected porous structure for cell seeding, proliferation and growth, as well as for filling bone defects (1). At the same time, they provide mechanical competence during bone regeneration. Biocompatibility, osteo-conductivity and/or inductivity, and suitable biodegradation rate are the properties required for a scaffold to be successful in bone tissue engineering. Scaffolds should also support attachment and proliferation of differentiating mesenchymal stem cells (MSCs) and osteoblasts (15) and therefore enhance bone formation and angiogenesis. MSCs are the most common source of osteoprogenitor cells and they are often derived from bone marrow (7, 16), adipose tissue (17), and other tissues such as periodontal tissue (18). The high proliferation rate and the multipotent differentiation potential of these cells qualify them for this purpose (19). However, a key role in the commitment and modulation of MSC activity towards bone regeneration is played by the presence of specific soluble mediators in the bone microenvironment. These are growth factors and cytokines, or insoluble extracellular matrix proteins, acting as paracrine regulators of stem-cell function, whose supplementation is mandatory in in vitro cultures (osteogenic media) (20-21-22).
Among biophysical stimuli, mechanical stimuli such as high- or low-intensity ultrasounds (HIFU or LIPUS) and pulsed electromagnetic fields (PEMF) were widely investigated in their ability to induce osteogenic differentiation (23-24-25-26-27-28-29-30). Most of these stimuli act on cell structures through a mechano-transduction mechanism, converting the stimulus into chemical signals. Both types of biophysical stimuli act in primis at cell membrane level, which represents the system able to translate an external signal into intracellular changes by activating several signal transduction pathways. In particular, PEMF induces an increase of intracellular Ca2+ from the endoplasmic reticulum with consequent increase in calmodulin, a protein known for stimulating nucleotides synthesis, cell proliferation and for inducing the production of growth factors (11). Changes in intracellular calcium, following the activation of ions channels are considered among the first cellular responses to mechanical stimuli (12). Conversely, it is not know which mechanosensitive membrane molecules such as ionic channels, G proteins coupled receptors, adhesion molecules and cytoskeleton components, are specifically activated by LIPUS (11, 31).
LIPUS has proven to be a clinically established, widely used and FDA approved therapy to enhance bone growth during healing of non-union fractures and other osseous defects (32, 33). Various studies have also shown that ultrasound can control the rate of scaffold degradation (13, 34-35-36), improve scaffold integration, and that they modulate different specific cellular aspects (14, 29, 37-38-39-40-41-42). Recently, our group demonstrated that LIPUS with spatial averaged and temporal averaged (SATA) intensity of 30 mW/cm2 is able (i) to maintain hMSCs stemness in vitro for up to 28 days and therefore guarantee the presence of a stem cells reservoir and (ii) to enhance and accelerate the osteogenic differentiation of hMSCs, thereby inducing the release of a master regulator of the angiogenic process (VEGF) (unpublished data).
Starting from the hypothesis that combined differentiating stimuli might accelerate the osteogenic differentiation of hMSCs, the aim of this study was to investigate the possible adjuvant effect of LIPUS stimulation on hMSCs seeded on an innovative biomimetic composite scaffold for bone regeneration, prepared with type I collagen and co-precipitated with bioactive magnesium-doped hydroxyapatite (Mg/HA) crystals (4, 43). The attention was focused on hMSCs osteogenic differentiating capability and colonization, through the analysis of osteogenic pathways, MAPK1/6 signaling related to cell stretch and compression, and IL-8 and VEGF expression in response to LIPUS stimulation.
Materials and Methods
MgHA/Coll hybrid composite scaffold
The scaffold (∅ = 6 mm, h = 5 mm) was manufactured by Fin-Ceramica Faenza SpA (Faenza – Ravenna, Italy). A 0.04 M H3PO4 solution was mixed with the aqueous acetic buffer solution of type I atelocollagen (1 wt%), which was then dropped into a basic suspension containing Ca(OH)2 0.04 M, MgCl2 6H2O (2 × 10−3 M) and simulated body fluid (SBF), yielding to a magnesium-HA/collagen material with a theoretical ratio of 70/30% and Mg/Ca molar ratio of 5% in the crystal lattice (44-45-46). Precipitate fibers were maturated for 1 hour, then washed with highly purified water and immediately submitted to a treatment of cross-linking by 48 hours’ immersion in NaHCO3/Na2CO3 buffer solution at pH = 9.5 of 1 wt% 1, 4-butanediol diglycidyl ether (BDDGE) cross-linking agent at 37°C (44). After the cross-linking reaction, the manufactured scaffold underwent a freeze-drying treatment consisting into a controlled freezing/heating ramp (from 25°C to 35°C, from 35°C to 20°C) carried out over 25 hours under vacuum conditions (0.29 mbar), to consolidate the 3D scaffold (MgHA/Coll hybrid composite) (46). Finally, MgHA/Coll hybrid composite scaffolds were packed separately and sterilized with γ radiation at 25 kGy.
Ethics statement
In this study, we used human mesenchymal stem cells (hMSCs, Lonza, Walkersville, MD USA) according to Lonza limited use license. Specifically, hMSCs were not used: a) in humans; b) in conjunction with human clinical trials; or c) in association with human diagnostics.
Cell culture
Human MSCs were cultured in mesenchymal stem cell growth medium (MSCGM™ Bullet Kit, Lonza, Walkersville, MD USA) to expand cells without inducing differentiation. The culture medium was changed every 3 days, and cells were split at 80%-90% of confluence using StemPro Accutase (Gibco by Life Technologies, Grand Islands, NY USA). To perform osteogenic differentiation, hMSCs were treated with hMSC mesenchymal stem cell osteogenic differentiation medium (OM) (hMSC Osteogenic Differentiation Bullet Kit™, Lonza).
Before cell seeding, MgHA/Coll hybrid composite scaffolds were pre-wetted in OM for 40 minutes to promote cell adhesion, hMSCs were then gently seeded onto them (25.000 cells/scaffold in 5 µL) carefully repeating cells deposition and recovery (cell engineered scaffold). This procedure let cell infiltrate into the porous structure, preventing cell dispersion. After 1 hour, each MgHA/Coll hybrid composite scaffold was carefully placed into a new 12-well plate (Costar, NY, USA) and covered with OM.
LIPUS treatment
The LIPUS exposure device manufactured by IGEA SpA (Carpi-Modena, Italy) consists of an array of 5 transducers (∅ 25 mm), which are specifically designed for use in a multiwell culture plate. LIPUS signal consisted of 200 μs burst of 1.5 MHz sine waves repeating at 1 kHz and delivering 30 mW/cm2 SATA intensity. A calibrated force balance measured the power of the collimated ultrasound beam emitted from the transducer, which was inserted in water perpendicularly to the measuring cone and in a concentric position relative to the latter (Ultrasound Power Meters UPM-DT-1AV, Ohmic Instruments, St. Charles – MI, US). By considering a probe value of effective radiating area of about 5.1 cm2, the mediated power was 33.7 mW/cm2. The wave form and frequency were measured using an oscilloscope (720A, Tektronix Inc., Beaverton - OR, US).
Twenty-four hours before LIPUS treatment, hMSCs cells were seeded onto the osteogenic scaffolds as described above. Cell cultures were divided in two groups for each experimental time (7 and 14 days): LIPUS-treated cultures (LIPUS scaffold) and untreated cultures (Untreated Scaffold). The culture plates were then placed on the ultrasound transducer array with a thin layer of standard ultrasound gel and exposed to LIPUS for 20 min/day for 5 consecutive days/week. The Untreated Scaffold group was handled in the same way, but the ultrasound generator was switched off. At the end of LIPUS stimulation time (14 days on), a culture plate for each group was maintained for further 7 days in the incubator at the same conditions, but without being exposed to the LIPUS device (indicated as ‘14 days on +7 days off’). In addition, osteogenic scaffolds without cells were cultured at the same conditions and used as negative controls.
dsDNA concentration (PicoGreen assay)
The concentration of dsDNA content was quantified by using fluorimetric Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen™, Life Technologies - EuroClone S.p.A, Pero-Milan, Italy). After scaffold washing with phosphate-buffered saline, 250 μL of lysis solution were added to each MgHA/Coll hybrid composite scaffold and cell lysis was then completed by 3 freeze-thaw cycles. After 5 minutes of incubation at room temperature (RT) and protected from light, dsDNA content was calculated from the lysates adding 100 μL of fluorescent nucleic acid stain to each scaffold (47). Fluorescence was measured using a GloMax multiwell plate reader (GloMax, Promega Corporation Madison, WI).
Scanning electron microscopy (SEM)
Both LIPUS Scaffolds and Untreated Scaffolds were fixed for 20 minutes in 2.5% glutaraldehyde in 0.1 saline buffer at pH 7.2, at RT to provide a rapid inter- and intra-cellular penetration and fixation, followed by post-fixation in saline buffer, with 3 changes for 10 minutes at RT. The fixed scaffolds were taken through a series of increasing concentrations of a drying ethanol solution (10%, 20%, 30%, 50%, 70%, 90%) ending in a 100% dehydrating liquid of the highest possible purity. After having carried out a critical point drier (K850 Critical Point Drier, Quorum Technologies LTD, Ashford UK – Assing SpA, Monterotondo-Roma, Italy), scaffolds were gold coated (B7340 Manual Sputter Coater Assing SpA) and then analyzed using a scanning electron microscope (EVO LS - ZEISS, Assing SpA). The backscattered electron observations were performed at 20 kV.
Reverse transcriptase - quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the scaffold using Trizol reagent (Invitrogen™). Each cDNA sample was tested in duplicate. Quantitative RT-PCR analysis was performed in a LightCycler 2.0 Instrument (Roche Diagnostics SpA, Milan, Italy) using SYBR® Green Real-Time PCR Master Mixes (Applied Biosystems™, Life Technologies - EuroClone S.p.A). QuantiTect Primers (Qiagen Srl, Milan, Italy) and designed primers (Invitrogen™) were used (Tab. I). Gene expression analysis was performed employing the 2−ΔΔCT method using GAPDH expression as reference gene (48). Results were expressed as relative fold changes calculated using Untreated Scaffold data as calibrator for each experimental time point.
Table I.
Gene primers specific for osteogenic differentiation or involved in the differentiating process. Expression was normalized versus GAPDH reference gene
| Gene | Forward primer | Reverse primer | Annealing temperature (°C) |
|---|---|---|---|
| RUNX2 | Hs_RUNX_l_SG | 60 | |
| ALPL | Hs_ALP_1_SG | 60 | |
| COL1A1 | Hs_COL1A1_l_SG | 60 | |
| BGLAP | Hs_BGLAP_1_SG | 60 | |
| SPP1 | Hs_SPP1_1_SG | 60 | |
| MAPK1 | GCGCTACACTAATCTCTCGT | CTGAGGTGCTGTGTCTTCAA | 60 |
| MAPK6 | GAATGGCAAATCTGCTCAATT | ACAGTCCTCCCCACCACTCA | 60 |
| VEGF | Hs_VEGFB_1_SG | 60 | |
| GAPDH | ATGGGGAAGGTGAAGGTCG | GGGTCATTGATGGCAACAATATC | 65 |
ELISA assays
Protein release in the culture medium for alkaline phosphatase (ALP), collagen type I alpha 1 (COL1a1), osteopontin (OPN), and the cellular content of osteocalcin (OC) were evaluated by Cloud-Clone ELISA kit (Cloud-Clone Corp. Houston, TX, USA), while interleukin 8 (IL8) (human IL8 ELISA KIT KHC0081) was evaluated by Invitrogen ELISA KIT assay (Invitrogen, Thermo Scientific, Italy). Values were normalized for medium protein content evaluated by Bradford assay.
Statistical Analysis
The results of each performed analysis was obtained by three independent experiments in replication. Statistical analysis was performed using the IBM® SPSS® Statistics 23 software. Results of LIPUS Scaffold group were expressed as mean ± standard deviation (SD) of increase (fold of increase - FOI) compared to Untreated Scaffold group at each experimental time and at a significance level of p<0.05.
After having verified the normal distribution (Kolmogorov-Smirnov test) and homoscedasticity (Levene test) of the data, one-way ANOVA, followed by adjusted Sidak's multiple comparison test, was performed to assess the influence of LIPUS treatment exposure on hMSCs osteogenic differentiation.
Results
Mg-HA/collagen porous composite scaffold
Physical-chemical characterization results of MgHA/Coll hybrid scaffold have been previously reported (44-45-46). SEM analysis showed that the Mg-HA/collagen porous composite scaffold presented a homogenous structure with tridimensional high porosity (83.8 ± 5.3%), a high degree of pore interconnectivity (mean size >100 µm) and evident large channel around 600 micron (44-45-46). EDS semiquantitative analysis of the elements contained in the MgHA/Coll hybrid composite scaffold showed a 0.32 ± 0.04 wt% for Mg, 20.31 ± 0.18 wt% for Ca and 10.08 ± 0.13 wt% for P (46). The mineral content analyses of this scaffold showed a strong interaction between the organic and inorganic (Mg-HA 50.5 ± 1.0 wt%) components, with the mineral phase structurally confined by the organic template and collagen enzymatic degradation completed in more than 5 months (44, 45). Transmission electron microscopy highlighted the enucleation of HA on collagen of Mg-HA/collagen porous composite scaffold and the presence of HA crystals inside the collagen matrix (46). Finally, inductively coupled plasma-optical emission spectrometry highlighted that 40 ± 1 w/w% Mg ions were released within one day and no significant differences in Mg ions release were found over 14 days (46).
Cell viability
To evaluate hMSCs viability and amount on MgHA/Coll hybrid composite scaffold, the PicoGreen® dsDNA quantification assay was used (Fig. 1). The LIPUS treatment did not alter dsDNA content on engineered osteogenic scaffolds and, after the end of treatment (14 days on +7 days off), an increase in dsDNA content (1.7 FOI) was found in the LIPUS Scaffold group compared to the Untreated Scaffold group (F = 53.66, p<0.0005, f = 0.55).
Fig. 1.
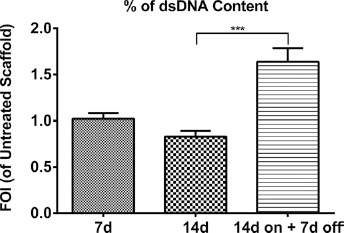
Amount of cells. DNA quantification of hMSCs seeded onto osteogenic scaffold and treated with LIPUS stimulation (LIPUS Scaffold) at each experimental time point, expressed as fold of increase (FOI) of Untreated Scaffold data (FOI = 1). Data are reported as mean±SD (n = 3, replicates). Adjusted Sidak's multiple comparison test:
*** p<0.0005.
Ultrastructural analysis
SEM analysis showed the capability of hMSCs to colonize the MgHA/Coll hybrid composite scaffold (Fig. 2). Untreated Scaffolds showed the same hMSCs colonization at every time point (Figs. 2A, 2C, 2E), confirming the data obtained by hMSCs viability analysis. Conversely, an increase of hMSCs colonization in LIPUS Scaffold group was more evident after 14 days of LIPUS treatment, remaining constant even after the LIPUS treatment was switched off (Figs. 2B, 2D, 2F).
Fig. 2.
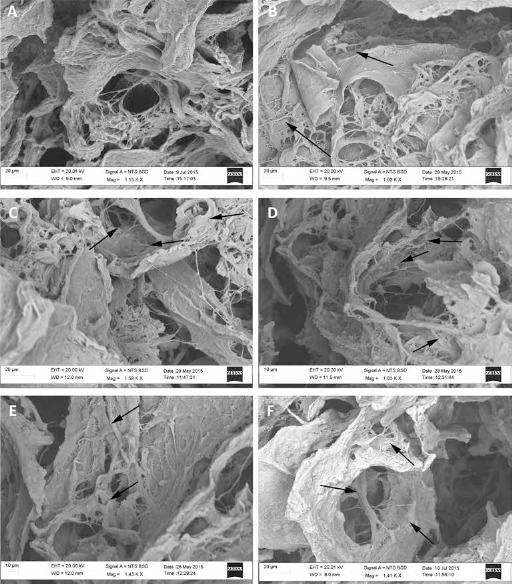
Ultrastructural analysis. SEM images of untreated (Untreated Scaffold: A, C and E) and LIPUS treated (LIPUS scaffold: B, D and F) engineered osteogenic scaffold at 7d (A and B), 14d (C and D), and 14 days on +7 days off (E and F) (scale bar: 10 µm). The arrows indicate hMSCs spreading onto the scaffold surface.
Gene expression
LIPUS treatment induced a gene expression modulation of several genes involved both in osteoblast differentiation (ALPL, COL1A1, BGLAP and SPP1), MAPK/ERK pathway (MAPK1 and MAPK6) and angiogenesis pathways (VEGF) (Fig. 3). In particular, LIPUS treatment produced: (i) no significant RUNX2 gene expression modulation; (ii) a constant increase of ALPL gene expression after 14 days of treatment (11.8 FOI), which grew further at 14 days on +7 days off (22.0 FOI) compared to the Untreated Scaffold group (F = 29.77, p<0.005, f = 0.58) and no modulation of COL1A1 compared to the Untreated Scaffold group (Fig. 3A); (iii) an increase of BGLAP (F = 65.65, p<0.0005, f = 0.57) gene expression at 14 days (1.58 FOI) (Fig. 3B); (iv) an increase of MAPK1 and MAPK6 expression compared to the Untreated Scaffold group: in detail, MAPK1 increased after 7 days of treatment (5.6 FOI) and remained constant over time (F = 0.22, NS), while MAPK6 increased at 14 days (4.9 FOI, F = 6.55, p<0.05, f = 0.52) (Fig. 3C); and finally (v) an increase in VEGF (F = 6.01, p<0.05, f = 0.54) expression in comparison with the Untreated Scaffold group, at 14 days (5.0 FOI), which remained constant up to 14 days on +7 days off of LIPUS treatment (Fig. 3C).
Fig. 3.
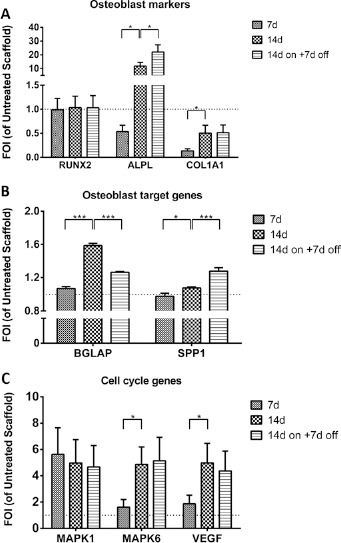
Gene expression analysis. Relative gene expression of osteoblast markers (RUNX2, ALPL and COL1A1), osteoblast target genes (BGLAP and SPP1) and cell cycle genes (MAPK1, MAPK6 and VEGF) in hMSCs seeded onto osteogenic scaffold and treated with LIPUS stimulation (LIPUS Scaffold) at each experimental time point, expressed as fold of increase (FOI) of Untreated Scaffold data (FOI = 1, dot line). Data are reported as mean ± SD (n = 3, replicates). Adjusted Sidak's multiple comparison test:
* p<0.05; *** p<0.0005.
Protein release
LIPUS stimuli did not induce an ALP release, and values stayed below those of the Untreated Scaffold group (Fig. 4). COL1a1 release was significantly higher at 7 days, decreasing below the values of the Untreated Scaffold group over time (F = 6.01, p<0.05, f = 0.54). OCN and OPN protein release showed an increase in comparison to the Untreated Scaffold group (OCN: FOI >5 and OPN: FOI >3), which remained constant over time (Fig. 4). LIPUS treatment caused an increase in IL8 release (3.5 FOI) at 7 days of treatment and at 14 days on +7 days off (F: 14.98, p<0.005, f = 0.88).
Fig. 4.
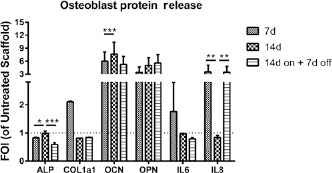
Protein release. ALP, COL1a1, OCN, OPN, and IL8 release by hMSCs seeded onto osteogenic scaffold and treated with LIPUS stimulation (LIPUS scaffold) at each experimental time point, expressed as fold of increase (FOI) of untreated scaffold data (FOI = 1, dot line). Data are reported as mean ± SD (n = 3, replicates). Adjusted Sidak's multiple comparison test:
* p<0.05; ** p<0.005; *** p<0.0005.
Discussion
The physical and chemical characteristics of a scaffold, as well as its osteointegration capability, have a fundamental role in the initial stage of bone regeneration. Nevertheless, it is necessary to guarantee hMSCs colonization into the scaffold, to commit hMSCs towards the osteoblastic lineage and to increase scaffold osteointegration capability through various strategies, including biophysical stimuli. The present study was carried out by using an innovative osteogenic scaffold – MgHA/Coll hybrid composite, whose physical and chemical characteristics, as well as its biocompatibility, had already been investigated (44, 46). Its fiber orientation, pore size and interconnectivity, together with the wettability of scaffold surfaces, could regulate cellular attachment and infiltration of the matrix, tuning the regeneration process. Natural polymers, such as collagen, are mechanically weaker, but flexible and usually contain specific molecular domains that induce and support cell bioactivity and biofunctionality.
Recent studies demonstrated that LIPUS stimuli transmit signals into the cell via an integrin that acts as a mechanoreceptor on the cell membrane (49). Other studies have proven that LIPUS treatment exerts a direct anabolic effect in osteoblasts, stimulating growth factors release, ALP activity, osteogenic differentiation, extracellular matrix production and accelerating calcium deposition (50). For these reasons, osteogenic-specific pathways modulation (ALP, COL1A1, RUNX2, BGLAP, SPP1), cell cycle (MAPK1 and MAPK6), angiogenetic (IL8 and VEGF) and inflammatory (IL6) specific factors were currently investigated.
The present results showed that LIPUS stimulation of hMSCs engineered scaffold can increase cell proliferation and MgHA/Coll hybrid composite scaffold colonization, in particular at 14 days on +7 days off of stimuli. This is probably due to the MAPK pathway activation, as highlighted by MAPK6 gene expression increase at 14 days and 14 days on +7 days off. MAPKs are serine/threonine kinases that regulate important cellular processes, including gene expression and cell proliferation, survival, death and motility (51). The role of MAPKs/ERKs in early stage differentiation of osteoblasts is currently debated, but many reports suggested that MAPKs activation is necessary for the maturation and mineralization of osteoblasts by inducing osteocalcin production (52, 53). Some studies support a stimulatory role in osteoblast differentiation, while others suggest that this pathway has an inhibitory role instead (54). The observed positive regulation of osteoblast late markers, such as OPN and OCN release in a time-dependent manner, suggested that LIPUS stimuli and MgHA/Coll hybrid composite scaffold might have a synergic role on hMSCs osteogenic differentiation, probably through MAPK pathway (52, 53). On the contrary, the absence of the early ALP marker modulation after LIPUS treatment did not highlight the same synergic role, suggesting the importance of MgHA/Coll hybrid composite scaffold in the early step of the differentiation process, whereas LIPUS treatment seems to act on the late differentiation step. BGLAP, showed only a little and biologically insignificant decrease of RNA expression, probably due to high levels of protein.
Data on VEGF gene expression demonstrated that there was an increase of VEGF gene expression in LIPUS Scaffold group after 14 days of stimulation, which remained after 7 days without treatment. MAPK pathway activation seems to be determined by mechanical stress on the cellular plasma membrane and cytoskeletal structures. Similarly, the biophysical effects of LIPUS induced intracellular signal transductions and gene transcriptions (55), leading to VEGF gene over expression (56). VEGF is highly expressed in osteoblastic precursor cells and known to stimulate bone formation. In the present study, LIPUS treatment caused an increase in IL8 release. It was reported that, during the osteogenic differentiation process, hMSCs are able to release IL8 to support development, differentiation and regeneration processes. IL8 signaling is also a mediator of the angiogenesis pathway in synergy with VEGF-a (55, 57-58-59). On the other hand, the maintenance of basal expression levels of IL6 by LIPUS treatment might suggest a decrease in bone resorption (60), whereas the up-regulation of IL8 might suggest the hypothetical activation of angiogenesis pathway after osteogenic differentiation stimuli (14 days on +7 days off) useful for bone engineering approach. For these reasons, the current IL8 and VEGF results support the hypothesis that LIPUS is able to stimulate angiogenesis.
In conclusion, the current study showed that the mechanical stimuli by LIPUS treatment improved colonization and differentiation of hMSCs seeded on a new biomimetic scaffold for bone regeneration. Based on these results, we think that LIPUS treatment might be applied to improve scaffold colonization and osteointegration acting as an adjuvant therapeutic approach useful to accelerate bone regeneration pathways.
Disclosures
Financial support: National Operational Programme for Research and Competitiveness 2007-2013 - PON01_00829 “Piattaforme Tecnologiche per l’Ingegneria Tissutale”; Operational Programme ERDF 2007-2013 in the region Emilia-Romagna: Activity 1.1 “Creation of technology centers for Industrial research and technological transfer”, and by Rizzoli Orthopedic Institute (“Cinque per mille 2012”).
Conflict of interest: No benefits in any form have been received or will be received from a commercial part related directly or indirectly to the subject of this article.
References
- 1.Murphy CM Haugh MG OBrien FJ The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 201031(3)461–466. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher DW Scaffolds in tissue engineering bone and cartilage. Biomaterials 200021(24)2529–2543. [DOI] [PubMed] [Google Scholar]
- 3.Minardi S Corradetti B Taraballi F et al. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 201562128–137. [DOI] [PubMed] [Google Scholar]
- 4.Tampieri A Sandri M Landi E et al. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 200829(26)3539–3546. [DOI] [PubMed] [Google Scholar]
- 5.Colnot C Cell sources for bone tissue engineering: insights from basic science. Tissue Eng Part B Rev 201117(6)449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvidson K Abdallah BM Applegate LA et al. Bone regeneration and stem cells. J Cell Mol Med 201115(4)718–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianco P Riminucci M Gronthos S Robey PG Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 200119(3)180–192. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 200511(7-8)1198–1211. [DOI] [PubMed] [Google Scholar]
- 9.Arinzeh TL Mesenchymal stem cells for bone repair: preclinical studies and potential orthopedic applications. Foot Ankle Clin 200510(4)651–665 viii.. [DOI] [PubMed] [Google Scholar]
- 10.Aaron RK Ciombor DM Wang S Simon B Clinical biophysics: the promotion of skeletal repair by physical forces. Ann N Y Acad Sci 20061068(1)513–531. [DOI] [PubMed] [Google Scholar]
- 11.Li JK Lin JC Liu HC et al. Comparison of ultrasound and electromagnetic field effects on osteoblast growth. Ultrasound Med Biol 200632(5)769–775. [DOI] [PubMed] [Google Scholar]
- 12.Brighton CT Wang W Seldes R Zhang G Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am 200183-A(10)1514–1523. [DOI] [PubMed] [Google Scholar]
- 13.Kim K Jeong CG Hollister SJ Non-invasive monitoring of tissue scaffold degradation using ultrasound elasticity imaging. Acta Biomater 20084(4)783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claes L Willie B The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 200793(1-3)384–398. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X Chang W Lee P et al. Polymer-ceramic spiral structured scaffolds for bone tissue engineering: effect of hydroxyapatite composition on human fetal osteoblasts. PLoS ONE 20149(1)e85871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominici M Le Blanc K Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 20068(4)315–317. [DOI] [PubMed] [Google Scholar]
- 17.Peterson B Zhang J Iglesias R et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 200511(1-2) 120–129. [DOI] [PubMed] [Google Scholar]
- 18.Huang GT Gronthos S Shi S Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 200988(9)792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detsch R Alles S Hum J et al. Osteogenic differentiation of umbilical cord and adipose derived stem cells onto highly porous 45S5 Bioglass®-based scaffolds. J Biomed Mater Res A 2015103(3)1029–1037. [DOI] [PubMed] [Google Scholar]
- 20.Lo KW Ulery BD Ashe KM Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev 201264(12)1277–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin MF Butler PE Seifalian AM Kalaskar DM Control of stem cell fate by engineering their micro and nanoenvironment. World J Stem Cells 20157(1)37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Q Dighe AS Irvine JN Jr. Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Curr Pharm Des 201319(19)3374–3383. [DOI] [PubMed] [Google Scholar]
- 23.Ongaro A Pellati A Bagheri L Fortini C Setti S De Mattei M Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells. Bioelectromagnetics 201435(6)426–436. [DOI] [PubMed] [Google Scholar]
- 24.Luo F Hou T Zhang Z Xie Z Wu X Xu J Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics 201235(4)e526–e531. [DOI] [PubMed] [Google Scholar]
- 25.Ceccarelli G Bloise N Mantelli M et al. A comparative analysis of the in vitro effects of pulsed electromagnetic field treatment on osteogenic differentiation of two different mesenchymal cell lineages. Biores Open Access 20132(4)283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson CG Martín-Saavedra FM Padilla F et al. Patterning expression of regenerative growth factors using high intensity focused ultrasound. Tissue Eng Part C Methods 201420(10)769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uddin SM Qin YX Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS ONE 20138(9)e73914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puts R Albers J Kadow-Romacker A Geissler S Raum K Influence of Donor Age and Stimulation Intensity on Osteogenic Differentiation of Rat Mesenchymal Stromal Cells in Response to Focused Low-Intensity Pulsed Ultrasound. Ultrasound Med Biol 201642(12)2965–2974. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y Peng W Liu X et al. Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound. Acta Biomater 201410(6)2518–2528. [DOI] [PubMed] [Google Scholar]
- 30.Kusuyama J Bandow K Shamoto M Kakimoto K Ohnishi T Matsuguchi T Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem 2014289(15)10330–10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato M Nagata K Kuroda S et al. Low-intensity pulsed ultrasound activates integrin-mediated mechanotransduction pathway in synovial cells. Ann Biomed Eng 201442(10)2156–2163. [DOI] [PubMed] [Google Scholar]
- 32.Nolte PA van der Krans A Patka P Janssen IM Ryaby JP Albers GH Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma 200151(4)693–702 discussion 702-703.. [DOI] [PubMed] [Google Scholar]
- 33.El-Mowafi H Mohsen M The effect of low-intensity pulsed ultrasound on callus maturation in tibial distraction osteogenesis. Int Orthop 200529(2)121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mather ML Crowe JA Morgan SP et al. Ultrasonic monitoring of foamed polymeric tissue scaffold fabrication. J Mater Sci Mater Med 200819(9)3071–3080. [DOI] [PubMed] [Google Scholar]
- 35.Parker NG Mather ML Morgan SP Povey MJ Longitudinal acoustic properties of poly(lactic acid) and poly(lactic-co-glycolic acid). Biomed Mater 20105(5)055004. [DOI] [PubMed] [Google Scholar]
- 36.Winterroth F Lee J Kuo S et al. Acoustic microscopy analyses to determine good vs. failed tissue engineered oral mucosa under normal or thermally stressed culture conditions. Ann Biomed Eng 201139(1)44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui JH Park SR Park K Choi BH Min BH Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng 200713(2)351–360. [DOI] [PubMed] [Google Scholar]
- 38.El-Bialy T Uludag H Jomha N Badylak SF In vivo ultrasound-assisted tissue-engineered mandibular condyle: a pilot study in rabbits. Tissue Eng Part C Methods 201016(6)1315–1323. [DOI] [PubMed] [Google Scholar]
- 39.Kruse DE Mackanos MA OConnell-Rodwell CE Contag CH Ferrara KW Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys Med Biol 200853(13)3641–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim K Kim J Seonwoo H Park SH Choung PH Chung JH In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering. Biomed Res Int 20132013269724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang MH Lim KT Choung PH Cho CS Chung JH Application of ultrasound stimulation in bone tissue engineering. Int J Stem Cells 20103(2)74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang SW Kuo CL Chang SJ et al. Does low-intensity pulsed ultrasound treatment repair articular cartilage injury? A rabbit model study. BMC Musculoskelet Disord 201415(1)36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landi E Logroscino G Proietti L Tampieri A Sandri M Sprio S Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J Mater Sci Mater Med 200819(1)239–247. [DOI] [PubMed] [Google Scholar]
- 44.Nicoletti A Fiorini M Paolillo J Dolcini L Sandri M Pressato D Effects of different crosslinking conditions on the chemical-physical properties of a novel bio-inspired composite scaffold stabilised with 1,4-butanediol diglycidyl ether (BDDGE). J Mater Sci Mater Med 201324(1)17–35. [DOI] [PubMed] [Google Scholar]
- 45.Calabrese G Giuffrida R Fabbi C et al. Collagen-hydroxyapatite scaffolds induce human adipose derived stem cells osteogenic differentiation in vitro. PLoS ONE 201611(3)e0151181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sartori M Pagani S Ferrari A et al. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater Sci Eng C Mater Biol Appl 201770(Pt 1)101–111. [DOI] [PubMed] [Google Scholar]
- 47.Ahn SJ Costa J Emanuel JR PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res 199624(13)2623–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 200125(4)402–408. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi R Ryo A Komitsu N et al. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis Res Ther 2008. 10(4)R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung KS Cheung WH Zhang C Lee KM Lo HK Low intensity pulsed ultrasound stimulates osteogenic activity of human periosteal cells. Clin Orthop Relat Res 2004418253–259. [DOI] [PubMed] [Google Scholar]
- 51.Chang L Karin M Mammalian MAP kinase signalling cascades. Nature 2001410(6824)37–40. [DOI] [PubMed] [Google Scholar]
- 52.Kuo PL Hsu YL Chang CH Chang JK Osthole-mediated cell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J Pharmacol Exp Ther 2005314(3)1290–1299. [DOI] [PubMed] [Google Scholar]
- 53.Tang N Song WX Luo J et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med 200913(8b 8B)2448–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schindeler A Little DG Osteoclasts but not osteoblasts are affected by a calcified surface treated with zoledronic acid in vitro. Biochem Biophys Res Commun 2005338(2)710–716. [DOI] [PubMed] [Google Scholar]
- 55.Khan Y Laurencin CT Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am 200890(Suppl 1)138–144. [DOI] [PubMed] [Google Scholar]
- 56.Hanawa K Ito K Aizawa K et al. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS ONE 20149(8)e104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Luca A Lamura L Gallo M Maffia V Normanno N Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J Cell Biochem 2012113(11)3363–3370. [DOI] [PubMed] [Google Scholar]
- 58.Liu CH Hwang SM Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 200532(6)270–279. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y Olsen BR Distinct VEGF functions during bone development and homeostasis. Arch Immunol Ther Exp (Warsz) 2014. 62(5)363–368. [DOI] [PubMed] [Google Scholar]
- 60.Axmann R Böhm C Krönke G Zwerina J Smolen J Schett G Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum 200960(9)2747–2756. [DOI] [PubMed] [Google Scholar]


