Abstract
Bothrops venezuelensis snake venoms, from five localities in the North-Central Venezuelan regions, showed biochemical and haemostatic differences. In this study, bioactivities of B. venezuelensis venoms from different regions (Aragua state; Waraira Repano (Capital District); Baruta, La Boyera and Lagunetica (Miranda state)) were compared using both natural and synthetic substrates. The protein contents of these venoms were Lagunetica 89%, La Boyera 79%, Baruta 71%, Waraira Repano 68% and Aragua 64%. Toxic activities effects were: Intraperitoneal LD50s: Aragua-14 mg/kg; Waraira Repano-6.4 mg/kg; Baruta: 8.3 mg/kg; La Boyera-4.4 mg/kg; Lagunetica-16.2 mg/kg. The MHD results: Aragua-21.4 μg/mouse; Waraira Repano-2.5 μg/mouse; Baruta-1.2 μg/mouse; La Boyera-1.4 μg/mouse and Lagunetica-12 μg/mouse. The hide powder azure results: Aragua-1.24 U/mg; La Boyera-2.26 U/mg; Baruta-2.83 U/mg; Lagunetica-3.28 U/mg and Waraira Repano-5.77 U/mg. Esterase specific activity on BAEE results: Waraira Repano-666.66 U/mg; La Boyera-805.5 U/mg; Baruta-900.00 U/mg; Lagunetica-922.19 U/mg and Aragua-1960.67 U/mg. Casein zymography showed digestion bands in the molecular weight above 100 and at 66.2 and 21.5 kDa. Analysis of casein degradation by SDS-PAGE showed two different degradation patterns. Fibrinolytic activity (mm2/μg) on fibrin plates results: Aragua-6.07; Lagunetica-27.6; Waraira Repano-35.7; La Boyera-44.27 and Baruta-45.63. In the fibrinogenolytic assay, the five venoms completely degraded the α chain after 1 min of incubation. None of the venoms completely degraded the β and γ chains after 24 h incubation. The research indicated that venoms of B. venezuelensis of different geographic areas in Venezuela exhibit variances in composition and component concentrations; except the Aragua venom, all of them had high proteolytic activities.
Keywords: Bothrops venezuelensis, Fibrinolytic, Fibrino(geno)lytic, Intraspecies variability, Proteolytic
1. Introduction
Bothrops snakes are geographically dispersed all over Venezuela from sea level up to 2500 m altitude (Rengifo and Rodríguez-Acosta, 2005) The Venezuelan tigra mariposa (Bothrops venezuelensis), whose name alludes to its aggressive nature and the butterfly-shaped pattern on its back, is broadly extended over the North-Western Venezuela across the Venezuelan Coastal and Andes Ranges, penetrating in its distribution towards Western Colombia (Sánchez et al., 2014). It is thought that this ample geographical distribution is one of the several factors that cause the existence of intraspecific variability venom composition, and their special effects, with consequent clinical and therapeutic implications (Chippaux et al., 1991).
Intraspecies venom variations have already been described for snakes coming from different geographical regions, and even from the same region. These venoms differ in their toxic activities and protein profiles, as has been demonstrated for Crotalus durissus cumanensis (Aguilar et al., 2007), Bothrops colombiensis (Girón et al., 2008), Bothrops atrox (Salazar et al., 2007) and Bothrops asper (Alape-Girón et al., 2008), among others. But, a comparative analysis of enzymatic and other toxic activities of venoms of Bothrops venezuelensis species found in different geographical areas of the Country had been lacking.
Bothrops venezuelensis envenomations are numerous and severe, characterised by immediate local effects and diverse systemic manifestations. A common local effect, at the bitten site, includes oedema, ecchymosis, compartmental syndrome, blisters, and necrosis. Systemic manifestations occur as spontaneous incoagulability and bleeding (haemorrhagic gums, haematuria, and epistaxis). Another important characteristic observed in these patients is the activation of the fibrinolytic system, with consumption of α2-antiplasmin and increase of fibrin(ogen) and D-dimer degradation products. These manifestations are associated with the presence of enzymes and non-enzymatic proteins in the venom, which produce clear effects on a victim’s haemostatic system (Sánchez et al., 2014).
The current work characterises the venoms of B. venezuelensis from distinctive Venezuelan geographical areas. Knowing the differences in their proteolytic activities as well as the variances among venom components influencing haemostasis would facilitate a better understanding of the clinical picture when humans are envenomed, and it could increase the understanding of antivenom specificity and reactivity, assuring the neutralization of the relevant pathological alterations caused by such diverse venoms.
2. Materials and methods
2.1. Reagents
Chromogenic substrates were obtained from Chromogenix AB (Milano, Italy). Molecular mass standards for SDS-PAGE were from Bio-Rad Laboratories Ltd. (California, USA). Purified factor Xa, bovine alpha thrombin, one chain t-PA (sctPA), Two chains u-PA (tcu-PA) and plasmin (used for controls), were obtained from American Diagnostica Inc. (Greenwich, CT, USA). Bovine fibrinogen (10% w/w of plasminogen as contaminant) was obtained from Sigma (St. Louis, MO, USA). Synthetic substrates, casein, hide powder azure, serine protease inhibitor cocktail, Na-Benzoyl-L-Arginine Ethyl Ester (BAEE) and other reagents used in this study were obtained from Sigma Chemical Co (St. Louis, MO, USA).
2.2. Animals
Albino Swiss NIH strain male mice of 18–22 g were purchased from the National Institute of Hygiene “Rafael Rangel,” Caracas, Venezuela. Animals were preserved under controlled light and environmental conditions with food and water ad libitum.
2.3. Snakes
Twenty-four (15 males and 9 females) Bothrops venezuelensis adult snakes, with distribution of males and females in each studied region, were captured during the Rainy Season (May to November) at Waraira Repano mountain (Capital District), Baruta, La Boyera and Lagunetica (Miranda state), and Maracay (Aragua state) in Venezuelan. All the snakes were caught in the north-central foot-hills regions of the Cordillera de la Costa range, between 10º29.2806’ - 10º14’07” North latitude and 66º52.7514’ - 67º35’28” West longitude, at 400–1500m above sea level. This area of origin of the specimens under study at the Cordillera de la Costa range presents a very similar type of climate influenced by the northeast trade winds, in the cloud forest of tropical floor, with average annual temperatures of 25 ºC and rainfall from 1300 to 1400mm; the vegetation is very similar to the valley with evergreen forests, mountains and semi-deciduous forests, its origin can be located in the tertiary geological era; forming part of the great Andean-American folding. The mountainous zone of the mountain range of the central coast is represented by sedimentary rocks with regional metamorphism and intrusive rocks (Pifano, 1961; Picard, 1976). Venoms were pooled samples from snakes (which included at least two males and two females in each pool) of the same location that were filtered through a 0.45-μm membrane, lyophilised, divided into 30 mg samples and stored at –80 ºC until used.
2.4. Lethal activity
The lethality of Bothrops venezuelensis venom was determined in five groups of 18–22 g mice containing five mice per group. The mice were injected via intraperitoneal with different venom concentrations (4.4–22.3 mg/kg; 1/1.5 dilutions) per group. The Lethal dose fifty (LD50) value calculated after 48 h, according to the Spearman-Kärber (1978) method.
2.5. Protein concentration determination
The protein concentration was determined by Lowry et al. (1951) method.
2.6. Chromatographic venom profiles
Samples from B. venezuelensis venom were run in a Superose 12 10/300 (General Electric, USA) molecular exclusion chromatography column, equilibrated with 50mM ammonium acetate pH 6.9 (equilibrium buffer). Venom samples (5 mg/100 μL) were diluted in equilibrium buffer and injected into the column. The elution was run with the same solution at 0.5 mL/min flow rate and monitored at 280 nm.
2.7. Polyacrylamide gel electrophoresis (SDS-PAGE) profiles of B. venezuelensis venoms from different Venezuelan locations
A total of 25 μg of each venom sample was evaluated by polyacrylamide gel electrophoresis (12.5%) in the presence of SDS, under reducing and non-reducing conditions (Laemmli, 1970), in a Mini-Protean II system (Bio-Rad Laboratories, Hercules, CA, USA). Briefly, venom samples were reconstituted in 0.063 M Tris-HCl buffer, pH 6.8, containing 2% SDS, 5% glycerol and 0.001% bromophenol blue and then boiled for 4 min before electrophoresis at 100 V (constant). After electrophoresis, the gels were stained with 0.1% brilliant blue Coomassie R250 in acetic acid/ethanol/water (5:25:70, v/v) and then distained in the same solution. The analysis was performed using the BioRad Quantity One 1-D Analysis Software Version 4.6.7 program.
2.8. Proteolytic activity
2.8.1. Casein zymography
A modified casein-substrate zymography was carried out with venom samples (40 μg) that were electrophoresed at room temperature in a 10% SDS gel containing 0.1% casein. After electrophoresis, gels were washed with 10mM Tris-HCl (pH 8.0) and 2.5% Triton X-100 and incubated at 37 ºC for 24 h in 50mM Tris-HCl (pH 8.0), containing 1% Triton X-100, 0.2 M NaCl, and 5mM CaCl2. Finally, gels were stained with 1% Coomassie Brilliant Blue.
2.8.2. Casein hydrolysis
The proteolytic activity of B. venezuelensis venom on casein was also evaluated by the detection of casein hydrolysis by SDS-PAGE electrophoresis on 12% gels, under reduced conditions (Laemmli, 1970). Briefly, specific cleavage on casein (Gay et al., 2005) by different B. venezuelensis samples was carried out using casein (5 mg/mL) heated at 100 ºC for 15 min in 0.1 M TriseHCl buffer (pH 8.0). Then, 20 μL of casein solution was incubated with 20 μL of venom samples at three different ratios of 100 μg:10 μg, 100 μg:5 μg, and 100 μg:1 μg (casein:venom) at 37 ºC for 30 min. A total of 40 μL of mixture was run on the gel at 20 mA.
2.8.3. Proteolytic activity of B. venezuelensis venoms on hide powder azure
A modified assay of Rinderknecht et al. (1968) was used. Hydrolysis of hide powder azure as established by incubating different amounts of venoms (100 μL; 0.8 mg/mL), dissolved in 2mL of 0.02 M Tris-HCl, pH 8.0, with 8 mg of hide powder azure for 1 h at 37 ºC and agitated at 5 min intervals. After incubation, each vial was centrifuged at 4000 × g for 5 min. Subsequently; the samples were transferred to different vials and measured at 595 nm. As a negative control, 0.02M Tris-HCl was used. The specific activity was expressed in U/mg protein, estimated as follows: change in absorbance in 1 h divided by mg of protein used.
2.8.4. Esterase activity of B. venezuelensis venoms on n-benzoyl-arginine ethyl ester (BAEE)
To quantify the activities of venom, trypsin and arginine esterase, the Tu et al. (1965) method on BAEE was used. This assay is based on the hydrolysis of the synthetic substrate BAEE by the action of the esterases present in the venom. BAEE and trypsin were tested as a positive control for the reaction. To achieve the enzymatic kinetics, the samples were read spectrophotometrically at 253 nm, every 30 s for 8 min and expressed in Units/mg, which is defined as the amount substrate in μmol/min converted to product by 1 mg of venom. The enzymatic activity was calculated by the formula:
where ΔA is the change in Absorbance.
Samples of B. venezuelensis venom (2 mg/mL) were incubated at 25 ºC, using 2.9 mL of 0.2mM BAEE in phosphate saline buffer (pH 7.0) and the absorbance was determined at 253 nm.
2.8.5. Gelatinase assay
A modified method of Ramírez et al. (1999) was used to test the gelatinase activity of B. venezuelensis venoms from different Venezuelan locations. The X-ray film (Kodak X-OMAT) was rinsed with distilled water and incubated at 37 ºC for 45 min. Following incubation, the film was entirely dried, and 25 μL (100 μg/25 μL) of each venom was placed on the X-ray scientific imaging film containing a gelatine coating. The hydrolysis of gelatine on the X-ray film was determined after 4 h incubation at 37 ºC, in a moist incubator by washing the X-ray film with distilled water. Serial dilutions were carried out to determine the minimum amount of venom necessary to produce a clearing area on the X-ray film. The titre was defined as the reciprocal of the highest dilution that caused a clear spot on the x-ray film. The specific gelatinase activity was determined by dividing the titre by the amount of protein (mg) applied on the film. The assay was repeated 3 times.
2.9. Minimal haemorrhagic dose (MHD)
Seven groups of mice (3 mice/group) were injected intradermically in the abdomen with various doses ranging from 1.25 μg to 80 μg (1/2 dilutions) of each B. venezuelensis venom dissolved in 0.1 mL PBS. Controls received 0.1 mL PBS under equal conditions. Three hours later, mice were euthanised by CO2 inhalation, the skin removed, and the haemorrhagic areas were measured. The minimum haemorrhagic dose (MHD) was the venom dose that induced a haemorrhagic area of 10mm diameter, according to the method of Gutiérrez et al. (1985). The MHD was obtained by constructing a dose-response curve. Each point on the graph represents the average of n = 3. The activity was adjusted to a positive and highly significant linear regression <0.01 for the localities of Aragua, Avila, Baruta, La Boyera and Lagunetica, respectively.
2.10. Coagulant activity
Coagulant activity of B. venezuelensis venoms was performed assaying six doses of each venom (3.13, 6.3, 12.5, 25.0, 50.0, and 100 μg/mL) to plasma, and between 25 and 500 μL for fibrinogen. A total of 100 mL of venom sample in saline was added to 200 μL of fibrinogen (Fg) or pre-incubated (37 ºC) human plasma obtained from healthy donors, and the clotting time registered. The minimum dose of venom that caused the coagulation of standardised solutions of fibrinogen (MCD-F), or human plasma (MCD-P) in 60 s at 37 ºC was determined to be the Minimum Coagulant Dose (MCD). The MCD-P and MCD-F were obtained by constructing a dose-response curve with a linear regression analysis. Each point on the graph represents the average of n 3. The activity was adjusted to a positive linear regression and highly significant, <0.01 for the MCD-P and <0.01 for the MCD-F for the localities of Aragua, Avila, Baruta, La Boyera and Lagunetica respectively (Theakston and Reid, 1983).
2.11. Amidolytic activity
Amidolytic activity of B. venezuelensis venom from different Venezuelan locations was measured by a micromethod standardised in our laboratory (Salazar et al., 2007). Briefly, in 96 polystyrene wells plates, a mixture of 80 μL of the recommended buffer for each substrate, 10 μL of the venom sample (0.1, 0.5 or 1 mg/mL) and 10 μL of chromogenic substrate were placed in each well. The final concentrations for the substrates were 0.6mM S-2238, 0.8mM S-2222, 0.8mM S-2251, 1.2mM S-2288, 0.16mM S-2444 and 4.0mM S-2302. Bovine thrombin, human factor Xa, plasmin, sct-PA, and tcu-PA were used as positive controls. After incubation at 37 ºC for 5 or 15 min, the absorbance at 405 nm was calculated. One unit of amidolytic activity was expressed as mUA 405 nm/min. Specific activity was calculated as mUA/min/mg.
2.12. Fibrinolytic activity
Fibrinolytic activities of B. venezuelensis snake venoms from different Venezuelan locations were tested by the fibrin plate method, as described by Marsh and Arocha-Piñango (1972). Briefly, fibrin plates were prepared in 3-cm diameter Petri dishes: 1.5mL of a 0.1% plasminogen-rich fibrinogen in imidazole saline buffer, pH 7.4 was clotted by adding 75 μL bovine thrombin (10 IU/mL, in 0.025 M CaCl2). The mixture was incubated at room temperature for 30 min. Then, a 10 μL (1 mg/mL) venom sample was applied over the fibrin film, and after 24 h incubation at 37 ºC, the diameters of the lysed areas were measured. Fibrinolytic activity was expressed as the diameter of the lysed area per μg of protein (mm2/mg). Human plasmin, t-PA (sct-PA) and u-PA (tcu-PA) were used as positive controls.
2.13. Fibrinogenolytic activity
The effect of B. venezuelensis snake venoms on fibrinogen chains was evaluated using a human fibrinogen solution. Briefly, fibrinogen was incubated with venoms at a 1 μg or 0.1 μg: 100 mg fibrinogen ratio at different times and at 37 ºC in presence or absence of serine protease inhibitor cocktails (benzamidine 10 mM, PMSF 1 mM, SBTI 1% and TLCK 50 μg/mL). The purpose of the inhibitor cocktails was to inhibit the coagulation allowing the visualization of the fibrinogenolytic activity since the coagulants in the venoms prevent the development of the assay. As controls, samples without protease inhibitors were used. The degradation of the fibrinogen chains was visualized on a 12.5% SDS-PAGE under reducing conditions using Tris-Tricine-system (Schägger and von Jagow, 1987).
2.14. Statistical analysis
The activities were described statistically according to their mean and standard deviation (Microsoft Excel). Variance analysis and the Student’s t-test were used to study possible differences between groups. For the analysis of masses and percentages in SDS-PAGE profiles of B. venezuelensis venoms were used the Quantity One 1-D Analysis Software version 4.6.7 (BioRad. USA). In the case of proteolytic activity on hide powder azure, Kruskal-Wallis tests and non-parametric Tukey a posteriori tests were applied. The statistical program STATISTICA version 7.0 was used. All statistical analyses were performed with a significance value of 0.05.
3. Results
3.1. Lethal activity
The LD50 from B. venezuelensis venoms from each studied region is shown in Table 1. The venom with the highest lethality was the specimens from La Boyera (4.4 mg/kg) and the least lethality came from Lagunetica (16.2 mg/kg).
Table 1:
Biological activities in venoms of B. venezuelensis from different Venezuelan locations.
| Locality | MHD (mg/mouse)a | Specific gelatinase activitya (U/mg) | LD50 (mg/kg) | Protein content (%) |
|---|---|---|---|---|
| Aragua | 21.4 ± 0.84 | 41.0 ± 1.76 | 14.0 | 64 |
| Waraira Repano | 2.5 ± 0.41 | 41.0 ± 1.25 | 6.4 | 68 |
| Baruta | 1.2 ± 0.34 | 655.4 ± 16.5 | 8.3 | 71 |
| La Boyera | 1.4 ± 0.40 | 2621.4 ± 38.4 | 4.4 | 79 |
| Lagunetica | 1.2 ± 0.45 | 10.2 ± 0.61 | 16.2 | 89 |
n = 3.
3.2. Minimal haemorrhagic dose (MHD)
The MHD from the venoms tested intradermal on mice is shown in Table 1. The Baruta venom presented the highest activity (1.2 μg/ mouse) and the lowest was Aragua (21.4 μg/mouse).
3.3. Protein concentration determination
Table 1 shows the average protein content, per milligram of the different venoms studied. The Lagunetica venom had the highest protein content followed by La Boyera and Baruta; the lowest concentrations belonged to the Waraira Repano and Aragua venoms.
3.4. Venom chromatographic venom profiles
The molecular exclusion column Superose 12 HR10/30 chromatographic profiles from the studied venoms are shown in Fig. 1. Eight highest peaks with different elution volumes (min) in all venoms were identified, which were classified according to their molecular mass as: very high (VH), high1 (H1), high2 (H2), intermediate1 (I1), intermediate2 (I2), medium (M), and low (L), respectively.
Fig. 1. Chromatographic profiles of B. venezuelensis venoms.
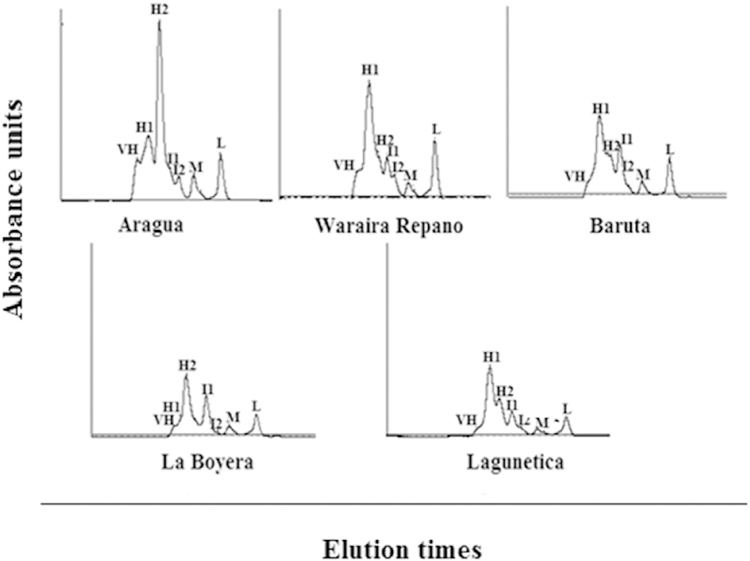
Samples of 5mg/100 μL were run in a Superose 12 10/300 GE column equilibrated with 50 nM ammonium acetate buffer, pH 6.9. The fractions were collected at a flow rate of 0.5 mL/min and monitored at 280 nm. The peaks were classified according to their molecular mass; VH: very high; H1: high1; H2: high2; I1: intermediate1; I2: intermediate2; M: medium; L: low.
3.5. Electrophoretic profiles of B. venezuelensis venoms
The proteins found were classified into five groups based on molecular masses: the first group corresponds to a molecular masses higher than 100 kDa; the second group between 100 and 66.2 kDa; the third group between 66.2 and 21, 5 kDa, the fourth group between 21.5 and 14.0 kDa, and the fifth group to proteins smaller than 14.0 kDa (Fig. 2). For the amount of material per band, expressed as a percentage (%), the analysis revealed that the venoms of Lagunetica, La Boyera, Baruta and Waraira Repano meant for masses of 54 kDa = 20–24%, for 27 kDa = 14–17% and for masses of approximately 16 kDa between 9 and 19%. In contrast, Aragua venom presented a fairly homogeneous distribution of its components, with a predominance of 9% for masses of 54 kDa, 16.3% for masses of 19.9 kDa and 15.5% for masses of 15.8 kDa.
Fig. 2. SDS polyacrylamide gel electrophoresis of B. venezuelensis venoms from different localities.
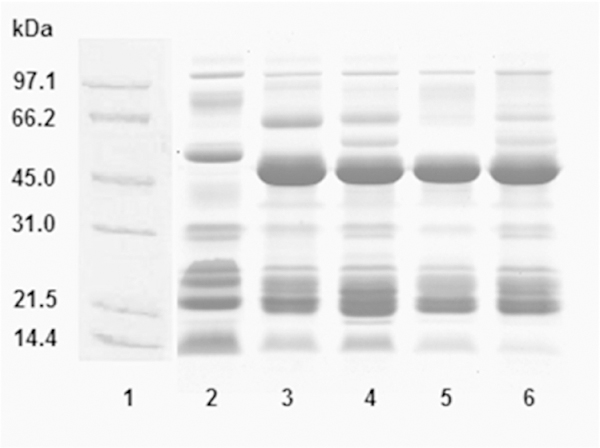
A total of 25 μg of each venom sample was evaluated by polyacrylamide gel electrophoresis (12.5%) in the presence of SDS, under reducing conditions in a Mini-Protean II system (Bio-Rad Laboratories, Hercules, CA, USA) at 100 V (constant). After electrophoresis, the gels were stained with 0.1% brilliant blue Coomassie R250 in acetic acid/ethanol/water (5:25:70, v/v) and then distained in the same solution. The analysis was performed using the BioRad Quantity One 1-D Analysis Software Version 4.6.7 program. Lanes: (1) molecular weight markers; (2) Aragua; (3) Waraira Repano; (4) Baruta; (5) La Boyera; (6) Lagunetica.
In gels under reducing conditions, generally fewer bands and smaller molecular masses were observed. The largest number of bands corresponds to Baruta venom and the smallest to the Waraira Repano venom. The venoms had in common, a range of bands from 50 to 43 kDa, not present in Aragua, and another between 25 and 21 kDa. Aragua venom had the higher content of material at low molecular masses (Fig. 2). Under non-reducing conditions (data not shown), for the amount of material per band (%), the analysis showed that Aragua venom had a more homogeneous distribution of its components with a maximum of 15.5% corresponding to 14.1 kDa. The venoms from the other four localities presented among them, analogous characteristics. Between 20 and 30% of the material corresponded to molecular masses of 47 to 46 kDa and between 11 and 18% the molecular masses matched up to a range of 23 to 21 kDa bands.
3.6. Proteolytic activity
3.6.1. Casein zymography of B. venezuelensis venoms
The zymogram (Fig. 3) shows the caseinolytic activity of B. venezuelensis venoms from different Venezuelan locations. The proteolytic activity was manifested as clear zones of the lysed substrate, which contrasted with the dark background. The zymogram showed caseinolytic activity in all the studied venoms. Digestion bands were observed in the high (100 kDa), intermediate (66.2 kDa) and low (21.5 kDa) molecular weights. Above 97.4 kDa, a diffuse degradation area was observed covering the most of the entire high molecular weight region. The highest enzyme content corresponds to the Aragua samples (Fig. 3, lane 6). This presents additional bands of digestion in the areas of molecular masses close to 45, 97.4 and 6.5 kDa.
Fig. 3. Casein zymographies of B. venezuelensis venoms from different localities.
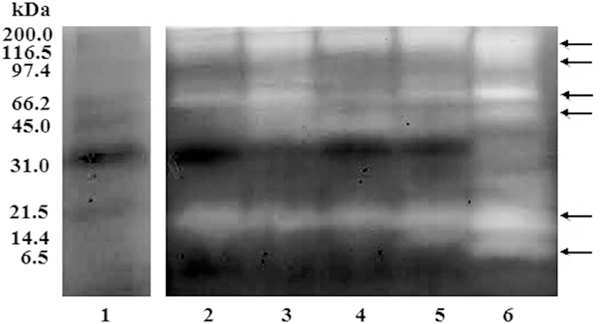
A total of 40 μg of venom samples were electrophoresed at room temperature in a 10% SDS gel containing 0.1% casein. After electrophoresis, gels were washed with 10mM Tris-HCl (pH 8.0) and 2.5% Triton X-100 and incubated at 37 ºC for 24 h in 50mM Tris-HCl (pH 8.0), containing 1% Triton X-100, 0.2M NaCl, and 5mM CaCl2. The gel was stained with 1% Coomassie Brilliant Blue. Lanes (1) Molecular weight markers; (2) Waraira Repano; (3) Baruta; (4) La Boyera; (5) Lagunetica; and (6) Aragua. The arrows indicate the locations representing white clear degradation areas.
3.6.2. Casein hydrolysis
The casein hydrolysis by Lagunetica and Aragua B. venezuelensis venoms showed two different results according with the venoms tested (Fig. 4A and B).
Fig. 4. Casein hydrolysis of B. venezuelensis-Lagunetica and Aragua venoms.
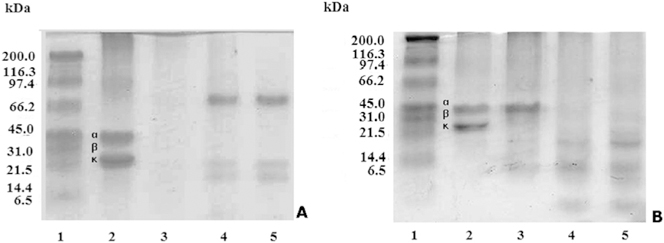
A total of 20 μL of a 5 mg/mL solution of casein was mixed with 20 mL of varying concentrations of venom giving a final ratio of 100 μg:10 μg, 100 μg:5 μg, 100 μg:1 μg (casein:venom). A total of 40 μL of mixture was run on a 12% SDS-PAGE at 20 mA for 1 h. A) Lagunetica venom. Lanes: (1) Molecular weight markers; (2) Casein; (3e5) Casein: venom at 100:10; 100:5; 100:1 ratios. B) Aragua venom. Lanes: (1) Molecular weight markers; (2) Casein; (3e5) Casein: venom at 100:10; 100:5; 100:1 ratios.
3.6.3. Proteolytic activity of B. venezuelensis venoms
The B. venezuelensis venoms’ proteolytic activities on hide powder azure substrate were expressed as specific activity in U/mg (Table 2). From lowest to highest activities were as follows: Aragua: 1.24; La Boyera: 2.26; Baruta: 2.83; Lagunetica: 3.28; Waraira Repano: 5.77 U/mg.
Table 2:
Proteolytic activity of B. venezuelensis venoms from different Venezuelan locations on hide powder azure and Na-Benzoyl-L-Arginine Ethyl Ester (BAEE) substrates (n = 3).
| Locality | Hide powder azure |
BAEE |
|---|---|---|
| Activity (U/mg) | Activity (mm2/μg) | |
| Aragua | 1.24 ± 0.03 | 1960.7 ± 42.8 |
| La Boyera | 2.26 ± 0.58 | 805.5 ± 67.3 |
| Baruta | 2.83 ± 0.44 | 900.0 ± 10.0 |
| Lagunetica | 3.28 ± 1.03 | 922.2 ± 58.5 |
| Waraira Repano | 5.77 ± 1.29 | 666.7 ± 63.1 |
3.6.4. Esterase activity of B. venezuelensis venoms
The B. venezuelensis venoms’ proteolytic activities on BAEE shown in Table 2 were expressed as specific activity in U/mg. Trypsin solution (591 U/mg activity) was used as positive control. The venom with the highest activity was Aragua (1960.7mm2/μg), and the least activity came from Waraira Repano (666.7mm2/μg).
3.6.5. Gelatinase assay
The specific gelatinase activities are shown in Table 1. The La Boyera venom presented the highest (2621.4 U/μg), and the lowest was Lagunetica (10.2 U/μg).
3.7. Coagulant activities
3.7.1. Amidolytic activity
As a result of the amidolytic method, using the chromogenic substrate S-2238 (Thrombin), Aragua venom was the most active. Using the S-2222 (factor Xa), the highest activity was Waraira Repano venom (Table 3).
Table 3:
Procoagulant activity in B. venezuelensis venoms from different Venezuelan locations (n = 3).
| Locality | Amidolytic method (mUA/min/mg) |
Coagulant method (μg) |
||
|---|---|---|---|---|
| S-2222 Factor Xa | S-2238 (Thrombin) | Plasma MCD-P | Purified Fibrinogen MCD-F | |
| Aragua | 40.6 ± 3.5 | 37.4 ± 6.8 | 0.6 ± 0.06 | 3.7 ± 0.43 |
| Waraira Repano | 37.1 ± 2.7 | 52.8 ± 3.7 | 0.7 ± 0.06 | 37.7 ± 1.42 |
| Baruta | 24.3 ± 0.8 | 32.4 ± 2.0 | 2.1 ± 0.39 | 25.5 ± 0.61 |
| La Boyera | 9.2 ± 1.8 | 10.0 ± 1.7 | 4.8 ± 0.47 | 11.3 ± 0.91 |
| Lagunetica | 17.3 ± 1.8 | 22.5 ± 0.7 | 1.9 ± 0.57 | 9.1 ± 0.53 |
3.7.2. Coagulant method
In the assays by the coagulant method in plasma and fibrinogen, the venom with the highest activity on plasma (MCD-P) was La Boyera (4.8 μg) and the least activity came from Aragua (0.6 μg). With respect to fibrinogen (MCD-F), Waraira Repano was the highest (37.7 μg), and the venom from Aragua was of the least active (3.7 μg) (Table 3).
3.8. Fibrinolytic activity
The B. venezuelensis venoms’ fibrinolytic activity was determined on fibrin plates and expressed as specific activity (mm2/μg) (Fig. 5 and Table 4). From lowest to highest were as follows: Aragua 6.07; Lagunetica 27.6; Waraira Repano 35.7; La Boyera 44.27 and Baruta 45.63mm2/μg.
Fig. 5. Fibrinolytic activity of B. venezuelensis venoms on fibrin plates.
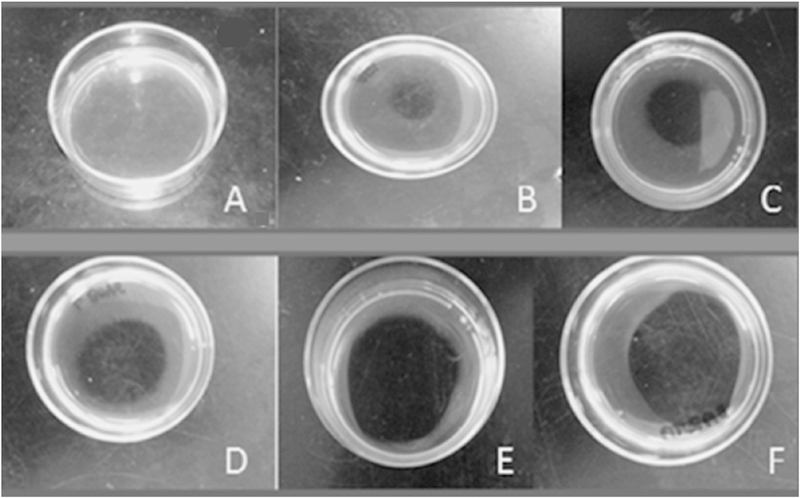
A) PBS control; B) Aragua; C) Lagunetica; D) Waraira Repano; E) La Boyera; F) Baruta.
Table 4.
Fibrinolytic activities of B. venezuelensis venoms from different Venezuelan locations (n = 3).
| Venom |
Fibrinolytic activities |
||||
|---|---|---|---|---|---|
| Locality | Fibrin plates |
Chromogenic substrates |
|||
| Plg (mm/mg) | (mUA/min/mg) |
||||
| S-2251 | S-2288 | S-2302 | S-2444 | ||
| Aragua | 7.3 ± 3.4 | 0.9 ± 0.13 | 68.7 ± 9.48 | 9.5 ± 1.05 | 11.1 ± 1.3 |
| Waraira Repano | 11.0 ± 4.7 | 1.2 ± 0.38 | 79.8 ± 1.98 | 6.2 ± 1.81 | 7.4 ± 1.4 |
| Baruta | 30.1 ± 8.9 | 0.3 ± 0.2 | 37.9 ± 4.1 | 3.1 ± 0.54 | 3.9 ± 0.7 |
| La Boyera | 9.5 ± 4.9 | 0 | 15.5 ± 0.42 | 1.5 ± 1.17 | 0 |
| Lagunetica | 33.7 ± 1.84 | 0.5 ± 0.12 | 27.2 ± 1.7 | 2.6 ± 0.2 | 0.4 ± 0.06 |
3.9. Fibrinogenolytic activity
Fig. 6 shows the effect of B. venezuelensis venoms on the fibrinogen chains. The control fibrinogen shows the three chains α, β and γ. The assay was performed at a 100:1 ratio (100 μg Fg/1 μg venom) for Aragua venom, since this venom did not have activity at 0.1 μg, and 100: 0.1 (100 μg Fg/0.1 μg venoms) for the rest of the venoms and at different incubation times. Only one representative gel with Lagunetica venom is shown (Fig. 6).
Fig. 6. Fibrinogenolytic activity of B. venezuelensis-Lagunetica venom.
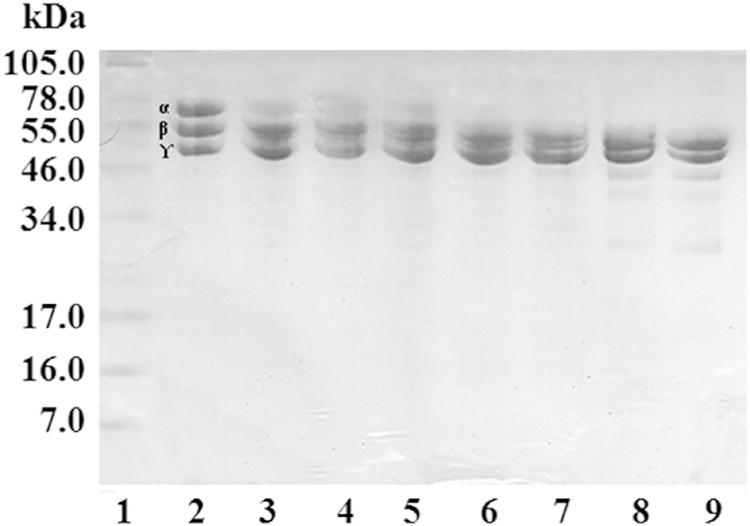
A mixture of 100 μg of fibrinogen with 0.1 μg of venom incubated at different times was run on a 12.5% Tris-tricine gel under reducing conditions. Lanes: 1) Molecular weight markers; 2) Fibrinogen (25 μg); 3) 1 min; 4) 15 min; 5) 30 min; 6) 1 h; 7) 2 h; 8) 4 h; 9) 24 h.
In the assays, performed initially without the inhibitory cocktail, all the venoms coagulated the fibrinogen before the incubation times ended. Aragua venom coagulated at 30s, Waraira Repano between 4 and 24 h, Baruta and La Boyera at 15 min and Lagunetica at 2 h.
When Waraira Repano B. venezuelensis venom was evaluated, degradation of the fibrinogen chains initiating with the Aα-chains began at 1 min and was completed at 2 h. Degradation of Bβ chains was initiated at 2 h, and degradation was still incomplete at 24 h. The degradation of γ chains started at 4 h and at 24 h had not yet been completed.
The degradation of Aα-chains by Lagunetica B. venezuelensis venom started at 1 min and was completed at 30 min. In the case of the Bβ chains, its degradation began at the 2 h and at 24 h part of these chains was still observed. The γ chains were not subjected to degradation during the 24 h of the assay.
Bothrops venezuelensis venom from Baruta initiated the degradation of the Aα chains at 1 min, which was complete at 30 min. Degradation of the Bβ chains started at the 2 h and not yet completed at 24 h. On the other hand, the degradation of γ chains began at 4 h still observing part of this at the 24 h.
In the case of La Boyera B. venezuelensis venom, the degradation of the Aα chains started at 1 min and was complete at 30 min. Its effect on the Bβ chains started at 2 h and could not be completed at 24 h. This venom initiated the degradation of the γ chains at 4 h, but was not complete during the 24 h of the assay.
The venom from Aragua, in the ratio of 100:0.1 (fibrinogen:venom) and at 24 h incubation did not induce degradation of the Aα, Bβ and ɤ chains. To demonstrate the fibrinogenolytic activity of this venom, the assay with a higher proportion of venom (100:1) was performed, observing the degradation of the Aα chains at 1 min and ending at 1 h, in the case of the Bβ chains the degradation began at 4 h, but could not be completed within 24 h. This venom did not degrade the ɤ chains.
4. Discussion
In Venezuela, snake bite envenomation is a Collective Health problem. Experts proposed an incidence of 7000 accidents per year, with 70–80% attributed to Bothrops snakes (Mota, 2008). Within this genus, Bothrops venezuelensis possesses the most potent venom (Sánchez et al., 2014). The regions chosen for the study, with an approximate extension of 4083 km2, can be seen in Fig. 7.
Fig. 7.
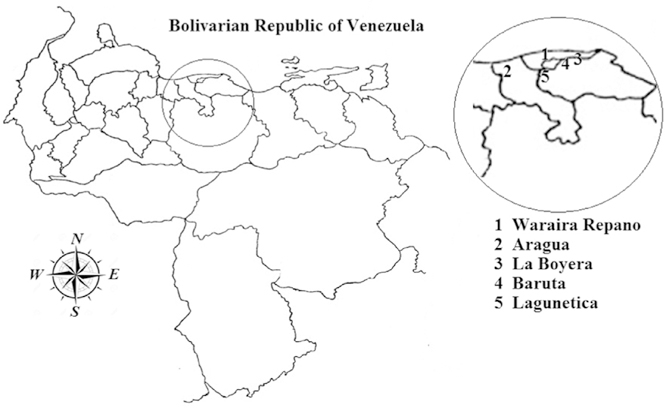
Geographical locations of the Bothrops venezuelensis venoms used in this study.
Single venom is a combination of toxins that generally act synergistically, in which context, composition varies between individuals of the same genus and, in some circumstances, within the same species. Numerous reports in the literature have documented different levels of variation in venom composition. Differences probably related to phylogenetic or taxonomic variances, age, size, geographical location, diet, seasonal variants and variations associated with sex, which lead to variations in the quantity and quality of the venom that it produces (Mackessy, 2002). It has also been determined that prolonged fasting (hibernation) affects the composition of the venom and makes the bite of a specimen in a post-hibernation state highly dangerous (Rengifo and Rodríguez-Acosta, 2005; De Sousa et al., 2013). These results offer further evidence that geographical isolation, natural selection, and adaptive evolution relating to feedings may be motivating forces assisting to population-level variation in venom composition (Saviola et al., 2017).
Various approaches were used to assess the diversity among venoms of B. venezuelensis from different geographical locations. Such methods have included processes linked to haemostasis, lethality and proteolytic activities caused by these venoms. Based on previous experiences confirming that variations occur between specimens within the same genus and species (Chippaux et al., 1991; López-Lozano et al., 2002; Aguilar et al., 2007; Salazar et al., 2007; Girón et al., 2008), the present study has been conducted to demonstrate that venoms of Bothrops venezuelensis from five different geographic areas of the central-northern region of Venezuela have intraspecies variability.
The intraperitoneally LD50 and haemorrhagic activities caused by B. venezuelensis are among the most potent in bothropic envenomations. Some authors (Lee and Lee, 1979) proposed that Bothrops venoms produced a critical fall in blood pressure, resulting in shock and later death. Bothropic venom consists of various structurally related polypeptides, such as bradykinin and kallidin and eicosanoids. These polypeptides act locally inducing vasodilation and contraction of smooth muscle resulting in the reduction of peripheral resistance (Markland et al., 1982), vascular permeability, and rupturing of the endothelial basement membrane, firstly induced by the haemorrhagings (Bjarnason and Fox, 1994). Despite that Lagunetica venom showed the least potent LD50 value but presented one of the most potent MHD value, does not indicate that death to animals bitten by these snakes is a result of the haemorrhagic action, but should correspond to the sum of toxic activities present in these venoms. Other activities present in these venoms consist of defibrinating actions, which are generated for non-haemorrhagic (procoagulant) venom proteases. These combined results lead to an extra reduction of the peripheral resistance, an intense reduction in cardiac output, and ultimately in irrevocable, hypovolemic shock (Lee and Lee, 1979). However, the Lagunetica venoms have a rather medium fibrinolytic activity, meaning that this action was not the most active in this pool venom; therefore its accountability in the death of the animals should be less. Regarding proteolytic activity, which could be involved in the lethality, only the gelatinase activity was lower in Lagunetica venom, being quite similar to the other proteolytic tests venoms.
From a clinical point of view, it was not easy comparing the venoms of the different regions because of the diversity of symptoms and activities caused by their cocktail of components. The venoms of all the regions studied displayed local and systemic haemorrhage as well as necrosis as the predominant activities; and therefore, it was not possible to obtain specific symptomatology information from the local physicians.
Chromatographic profiles of B. venezuelensis venoms are shown, and quantitative differences amid the venoms were demonstrated. The chromatography peaks showed the highest protein concentration in Aragua and Waraira Repano venoms. Lagunetica, La Boyera, and Baruta showed a lower concentration of H1 and H2 peaks. Aragua, Waraira Repano, Baruta, and Lagunetica were comparable due to the presence of intermediate protein concentrations in most of the peaks. La Boyera and Lagunetica had slightly lower peaks (L) than Aragua, Waraira Repano, and Baruta. The main interest in showing these chromatograms is to provide proof of the significant variation in these venoms.
Electrophoretic profiles of B. venezuelensis venoms from the five localities showed variations in their venom compositions, such as number and intensity of protein bands, which supported the differences in the biological activities evaluated. Regarding the amount of material per band (%), the analysis showed that Aragua venom had a more homogenous distribution of its components, with a maximum of 15.5% corresponding to 14.1 kDa under reducing and non-reducing conditions. The venoms from other four localities presented similar characteristics among them: between 20 and 30% of the material corresponded to molecular masses of 47e46 kDa and amid 11 and 18% to molecular masses in a range of bands 23e21. These results agree with observations carried out by others authors. For instance, B. alcatraz venom in Brazil (Furtado, 2005) and B. atrox venoms in Venezuela (Salazar et al., 2007) showed venom differences among distinct geographical areas. In the chromatographic analysis and electrophoretic profiles, differences in the molecular masses were found. Other differences, in lethality, haemorrhagic, fibrinolytic and coagulant activities, were also reported. Alternatively, Girón et al. (2008), comparing venoms of B. colombiensis from two localities of Miranda state (El Guapo and Caucagua), reported differences in their lethal, haemorrhagic, procoagulant, and fibrinogenolytic activities as well as in their electrophoretic and chromatographic profiles. Therein they describe that most components of Bothrops genus venoms were in the range of 64 to 14 kDa and possibly corresponded to metalloproteases (64–40 kDa), serine proteases (40–20 kDa) and phospholipases A2 (16–13 kDa).
Protein concentration differences in the venoms of this study were noticed, ranging from 89% (Lagunetica) to 64% (Aragua). Other authors have reported similar results with Peruvian species of B. atrox and B. pictus, with percentages of 68% and 66%, respectively (Sánchez et al., 2010). In contrast, Ortiz et al. (2012) working with B. atrox venom from different regions of Peru did not find significant differences in the quantity of protein. But, all venoms had high protein content (between 94 and 95%).
Some venom proteolytic enzymes also degraded tissue substrates such as gelatine, which has been a tool to illustrate both metalloproteases and serine proteinases activities (Shannon et al., 1989; Serrano and Maroun, 2005). The proteolytic activity on gelatine was observed in all evaluated B. venezuelensis venoms (U/μg), showing that the highest specific activity was found in La Boyera, followed by Baruta venom.
In order to continue comparing proteolytic activities of B. venezuelensis venoms, various synthetic and natural substrates were used. Synthetic substrates included hide powder azure and BAEE. The hide powder azure method is a quantitative, fast, reproducible, economical method that emerged as a substitute for gelatine in the evaluation of proteases activity. It maintains a linear relationship between the hydrolysis efficiency and the enzyme concentration or enzymatic activity (Rinderknecht et al., 1968).
In the present study, the activity of all venoms on hide powder azure was observed, demonstrating the action of a trypsin-like enzyme, with a higher specific activity in the locality of Waraira Repano, followed by Lagunetica, Baruta, and La Boyera; the lowest specific activity was found in venom of Aragua locality.
In relation to the proteolytic activity on the BAEE substrate used to also evaluate the trypsin-like activity, the study revealed that a higher activity of esterases was present in the venom of Aragua locality, followed by Lagunetica, Baruta, La Boyera, and showing less activity in the locality of Waraira Repano. Previously, López et al. (1999), characterizing venom of B. venezuelensis from specimens collected in Waraira Repano, reported an activity of 209 U/mg with BAEE, which was 3.2 times lower than those found in the current work.
The components of snake venoms, acting on the coagulation system, may have a direct effect on coagulation factors by specific proteolytic activation, or non-specific degradation. The coagulant activity is due to enzymes acting on plasma procoagulant components, for example factor IX, factor X and prothrombin activators, or by the action of thrombin-like enzymes. Bothropic venoms have high pro and anti-coagulant activities. These actions were associated with serine proteases (Castro et al., 2004; Markland, 1998).
Assessing the B. venezuelensis venom effect on haemostasis, the coagulant activity was studied on human plasma and chromogenic substrates, determining the presence of factor Xa and thrombin like activities. The venom from Waraira Repano presented a thrombin-like coagulant activity that was higher with low doses of venom (52.8 mUA/min/mg), indicating the presence of inhibitors acting on thrombin and/or factor Xa. In earlier works with Bothrops venoms, this coagulant activity was associated with thrombin-like enzymes, which acted by releasing fibrinogen A or B peptides, factor IX or factor X, and prothrombin activators, which can induce both platelet and coagulation cascade activation themselves. This may also explain the Disseminated Intravascular Coagulation cases that have been observed in victims of snakebite accidents, a condition that can conclude in bleeding by defibrination (Markland, 1998; Oyama and Takahashi, 2007; Larréché et al., 2008; Demler et al., 2010).
In the case of La Boyera B. venezuelensis venom, which had the highest activity on plasma, suggested that this venom has a higher variety of serine protease enzymes, related to the abundance of bands observed in the electrophoretic pattern and associated with disorders of coagulation. Highlighting the most procoagulant venom that had the highest direct coagulant effect and differentiating the activity on plasma and Fg was observed that Waraira Repano had the highest activity on Fg (37.7 μg) (Table 3).
In snake venoms there are components capable of activating the fibrinolytic system, or presenting enzymes with direct fibrinolytic action. These enzymes act on fibrinogen and fibrin, leading to blood defibrinogenation, fibrin lyses, and the consequent decrease in blood viscosity, which represents a mechanism to induce haemorrhagic manifestations in their victims. These venom components can be classified as α or β fibrinogenases, depending on their activity, preferably on one or the other chains of fibrinogen, and belong to the group of enzymes such as metalloproteases or serine proteases (Markland, 1998). The present comparative study of the fibrinolytic activity, evaluated by fibrin plate method, also showed obvious differences between the venoms. The highest fibrinolytic activity, expressed as specific activity (mm2/μg), was presented by Baruta venom-45.63, followed by La Boyera-44.27; Waraira Repano-35.7; Lagunetica-27.6, and Aragua-6.07. This direct venom action on fibrin could be due to enzymes, such as metalloproteases. Salazar et al. (2007), comparing venoms of B. atrox from different locations in southeast Venezuela, report the highest fibrinolytic activity of venom from Puerto Ayacucho-1 with 30.13mm2/μg. Girón et al. (2008) comparing B. colombiensis venoms from two different localities in Miranda state (Venezuela) reported the highest activity in Caucagua 30.6mm2/mg. Rodríguez-Acosta et al. (2010) characterizing B. isabelae venom from specimens collected at Trujillo state (Venezuela), reported a fibrinolytic activity of 500 mm/μg. Comparing the fibrinolytic activity values from B. venezuelenis venoms of Baruta, La Boyera and Lagunetica localities, with those reported for B. atrox, B. colombiensis and B. isabelae venoms, B. isabelae venom displays an overwhelmingly result.
The action of venoms on the fibrinogen chains showed that the highest activity was principally over Aa chains, which in the majority of the venoms started at 1 min of incubation. In order to see this proteolytic activity in Aragua, it was necessary to increase the concentration of venom incubated with the substrate, because the lowest fibrinogenolytic activity corresponded to this venom. None of the venoms were able to completely digest the Bb or g chains.
Most of the fibrino(geno)lytic enzymes are metalloproteases with specificity directed preferentially to the Aα chains of fibrinogen and with low activity on the Bβ chains. Serine proteases have fibrino(geno)lytic activity preferentially directed to the Bb chains of fibrinogen and with low activity on the Aα chains. Yet, some serine proteases have both fibrinogenolytic and fibrinolytic activities (Larrèché et al., 2008). In our study, this fibrino(geno)lytic activities were associated with metalloprotease-like enzymes, as verified in the development of these tests, because they were carried out in the presence of serine proteases inhibitors. As shown, B. venezuelensis venoms from the five localities contain enzymes that act on fibrinogen and fibrin could lead to defibrinogenation, fibrin lysis, and a decrease in blood viscosity in an envenomed individual. It is important to emphasize that these venoms contained high fibrinogenolytic activity in conjunction with high coagulant activity rendering them significantly pro-coagulant venoms.
The proteolytic activity of B. venezuelensis venom on casein was evaluated by zymography and detection of casein hydrolysis by SDS-PAGE electrophoresis on 12% gels. Concerning zymography, bands (˃100 kDa), intermediate (66.2 kDa) and low (21.5 kDa) weights were found. Molecular masses above 97.4 kDa, in Aragua samples showed a diffuse degradation area, covering the entire high molecular weight region. In relation to the degradation of casein detected on polyacrylamide gels, there were two distinct degradation patterns. For all venoms, the three chains (a, b and k) of casein were degraded. Degradation products were observed in bands of molecular masses between 70 and 60 kDa and between 21.5 and 14.4 kDa at 100: 5 and 100: 1 ratios. Studying the proteolytic activity of B. alternatus venom on casein, similar results were presented by Gay et al. (2005). In the present study, venoms of different geographical areas possessed very potent proteolytic activities, and they differed slightly from each other, except for the Aragua sample, which in all the trials showed a totally different behaviour.
All herein results appear to be a part of the intraspecific variations of snake venoms, which could be due to different evolutionary pressures, the availability of breeding pairs (by a possible hybridization of species), different types of prey as well as habitat. Alternatively, snakes might be exposed to selective forces during adaptation (Pizzatto et al., 2007), particularly in venomous snakes, in which the pressure is to improve its capacities to produce components of venom that aid in the digestion of the prey and in some circumstances for defence against predators.
The region of origin of our B. venezuelensis snake samples may propose that genomic distances between snake species have been influenced by genetic exchange between nearby ancestral populations, producing a genetic intraspecies distinctiveness variation, which has substantial heritability and involves qualities that are sufficiently common that they can be observed in any restrained-sized sampling of individuals. This current study suggested that genetic individuality is basically formed by the combinatory inheritance of a discreet number of genes, each of which exists as a family of functionally and structurally diverged alleles. Alternatively, mutation rates differ between evolutionary lineages (Bromham, 2009), they usually do not differ significantly enough to alter intraspecies variability. The difference here is based on how much mutation can occur within a set amount of time. Furthermore, there is known a process called canalisation, which is a degree of the capability of a population to generate the same phenotype, regardless of variability of its environment or genotype. Some species also develop plenty of variation due to biogeographically separations. For instance, if a species has a very large or fragmented distribution surviving in an extensive variety of different ecosystems, the populations of that species may differ, if there is no significant gene flow between these populations. Smaller species populations, more connected and more homogeneous ranges with probably remain more genetically and/or phenotypically homogeneous (Woolfit et al., 2010). Madritch and Hunter (2002) are persuaded that conserving genetic diversity within a species is as important as conserving species diversity for maintaining ecosystem functions.
5. Conclusions
The present study represents the first characterisation of intraspecific variations of the venom profiles and proteolytic activities of B. venezuelensis species. These findings allow us to deepen the understanding of the biology of this snake species, as well as the pathology induced by their venom, allowing establishing similarities and differences, which could be used for the development of new therapeutics and more effective treatments. These findings along with previous studies have to be considered in the development of antivenoms, and the pool used as venom references has to be obtained from a relatively large number of specimens collected in different geographical regions. Accepting the significance of these parameters allows the understanding of the value that venom variability at the intra-specific level has. After all, the success of antivenom therapeutics depends on it.
Acknowledgements
Funding for the project was provided by the Science and Technology Fund (FONACIT) programs (PG-2005000400) and the NIH/ ORIP, Viper Resource Grant #3P40OD010960–10S1, 2P40OD010960–11A1, and 5P40OD010960 (NNTRC, Texas A&M University-Kingsville, Dr. E.E. Sánchez). We would also like to thank two anonymous referees that helped to improve our manuscript, Lic. Carlos Ibarra for his technical assistance in the chromatography system operation and Nora Diaz De Leon (NNTRC).
Footnotes
Conflict of interest and disclosure
The authors declare that they have no conflict of interest. The authors confirm that there are no financial disclosures for this study.
Ethical statement
Proficient personnel prepared all the experimental procedures relating to the use of live animals according to the Venezuelan pertinent regulations and institutional guidelines for the care and use of laboratory animals, were published by the US National Institute of Health (Anonymous, 1985) and approved by the Institute of Anatomy Ethical Committee of the Universidad Central de Venezuela.
References
- Aguilar I, Guerrero B, Salazar AM, Girón ME, Pérez JC, Sa´nchez EE, Rodríguez-Acosta A, 2007. Individual venom variability in the South American rattlesnake Crotalus durissus cumanensis. Toxicon 50, 214–224. [DOI] [PubMed] [Google Scholar]
- Alape-Girón A, Sanz L, Escolano J, Flores-Díaz M, Madrigal M, Sasa M, Calvete JJ, 2008. Snake venomics of the lancehead pitviper Bothrops asper: geographic, individual, and ontogenetic variations. J. Proteome Res 7, 3556–3571. [DOI] [PubMed] [Google Scholar]
- Anonymous, 1985. National Institute of Health Principles of Laboratory Animal Care, USA, pp. 1–112. Pub. 85 No. 23. [Google Scholar]
- Bjarnason B, Fox JW, 1994. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther 62, 325–372. [DOI] [PubMed] [Google Scholar]
- Bromham L, 2009. Why do species vary in their rate of molecular evolution? Biol. Lett 5, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro HC, Zingali RB, Albuquerque MG, Pujol-Luz M, Rodrigues CR, 2004. Snake venom thrombin-like enzymes: from reptilase to now. Cell. Mol. Life Sci 6, 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J, Williams PV, White J, 1991. Snake venom variability: methods of study, results and interpretation. Toxicon 29, 1279–1303. [DOI] [PubMed] [Google Scholar]
- Demler C, Bühler B, Menin L, Stöcklin R, Wilmer M, Ernst B, Perchuc AM, 2010. Platelet-active substances in the venom of Bothrops moojeni snake-a novel evaluation method using whole blood aggregometry. Toxicon 21, 20–28. [DOI] [PubMed] [Google Scholar]
- De Sousa l., Bastouri J, Matos M, Borges A, Bónoli S, Vásquez A, Guerrero B, Rodríguez-Acosta A, 2013. Epidemiología del ofidismo en Venezuela (1996–2004). Invest. Clin 54, 123–137. [PubMed] [Google Scholar]
- Furtado MF, 2005. Biological and immunological properties of the venom of Bothrops alcatraz, an endemic species of pitviper from Brazil. Comp. Biochem. Physiol. C Toxicol. Pharmacol 141, 117–123. [DOI] [PubMed] [Google Scholar]
- Gay CC, Leiva LC, Maruñak S, Teibler P, Acosta de Pérez O., 2005. Proteolytic, edematogenic and myotoxic activities of a hemorrhagic metalloproteinase isolated from Bothrops alternatus venom. Toxicon 46, 546–554. [DOI] [PubMed] [Google Scholar]
- Girón M, Salazar A, Aguilar I, Pérez J, Sánchez EE, Arocha-Pinango C, Rodríguez-Acosta A, Guerrero B, 2008. Hemorrhagic, coagulant and fibrino(geno) litic activities of crude venom and fractions from mapanare (Bothrops colombiensis) snakes. Comp. Biochem. Physiol. C Toxicol. Pharmacol 147, 113–121. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Gene JA, Rojas G, Cerdas L, 1985. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 23, 887–893. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Larrèché S, Mion G, Goyffon M, 2008. Haemostasis disorders caused by snake venoms. Ann. Fr. Anesth. Reanim 27, 302–309. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee CY, 1979. Cardiovascular actions of snake venoms. In: Lee CY (Ed.), Handbook of Experimental Pharmacology, vol. 52 Springer-Verlag, Berlin, pp. 547–590. [Google Scholar]
- López JC, Vargas A, Scannone H, Fernandez Y, 1999. Estudio cromatográfico, electroforético y enzimático del veneno total y fracción I de la serpiente venezolana Bothrops venezuelensis (Tigra Mariposa). Rev. Cient. (Maracaibo) 4, 341–320. [Google Scholar]
- López-Lozano JL, Valle de Sousa M.V., Ricart CA, Chávez-Olortegui C, Flores Sánchez E., Muñiz EG, Bührnheim PF, Morhy L, 2002. Ontogenic variation of metalloproteinases and plasma coagulant activity in venoms of wild Bothrops atrox specimens from Amazonian rain forest. Toxicon 40, 997–1006. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosembrough NJ, Farr AL, Randall RJ, 1951. Protein measurement with the. Folin phenol reagent. J. Biol. Chem 193, 265–275. [PubMed] [Google Scholar]
- Mackessy SP, 2002. In: Stephen P, Mackessy (Eds.), Handbook of Venom and Toxins of Reptiles CRC Press. Taylor & Francis Group, Boca Raton-London-New York. [Google Scholar]
- Madritch DM, Hunter MD, 2002. Phenotypic diversity influences ecosystem functioning in an oak sandhills community. Ecology 83, 2084–2090. [Google Scholar]
- Markland FS, Kettner C, Schiffman S, Shaw E, Bajwa SS, Reddy KNN, Kirakossian H, Patkos GB, Theodor I, Pirkle R, 1982. Kallikrein-like activity of crotalase, a snake venom enzyme that clots fibrinogen. Proc. Natl. Acad. Sci. USA 79, 1688–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland FS, 1998. Snake venoms and the hemostatic system. Toxicon 36, 1749–1800. [DOI] [PubMed] [Google Scholar]
- Marsh NA, Arocha-Piñango CL, 1972. Evaluation of the fibrin plate method for estimating plasminogen activator. Thromb. Diath. Haemorrh 28, 75–88. [PubMed] [Google Scholar]
- Mota JV, 2008. Accidente ofídico en Venezuela. Universidad Rómulo Gallegos. Área de Ciencia de la Salud Centro de Rotaciones Asistenciales Hospital General Dr. “Victorino Santaella Ruiz”, Los Teques Estado Miranda, República Bolivariana de Venezuela. [Google Scholar]
- Ortiz C, Lazo F, Bellido C, Gonzales E, Yarlequé A, 2012. Variaciones en las actividades enzimáticas del veneno de la serpiente Bothrops atrox “jergo´n”, de tres zonas geográficas del Perú. Rev. Peru. Med. Exp. Sal. Púb 29, 198–205. [PubMed] [Google Scholar]
- Oyama E, Takahashi H, 2007. Distribution of low molecular weight platelet aggregation inhibitors from snake venoms. Toxicon 49, 293–298. [DOI] [PubMed] [Google Scholar]
- Picard X, 1976. Geología de la cuenca de Guarenas-Guatire, sedimentación continental intracordillerana, Venezuela. Memorias Congreso Latinoamericano Geol. II, Caracas, noviembre 1973. Bol. Geol. Publ. Espec 7 (II), 965–984. [Google Scholar]
- Pifano F, 1961. Investigación y docencia en Medicina Tropical. Arch. Venez. Med. Trop. Parasitol. Med 4, 1–203. [Google Scholar]
- Pizzatto L, Almeida-Santos SM, Shine R, 2007. Life-history adaptations to arboreality in snakes. Ecology 88, 359–366. [DOI] [PubMed] [Google Scholar]
- Ramírez MS, Sánchez EE, García-Prieto C, Pérez JC, Chapa GR, McKeller MR, Ramírez R, De Anda Y, 1999. Screening for fibrinolytic activity in eight Viperid venoms. Comp. Biochem. Physiol 124, 91–98. [DOI] [PubMed] [Google Scholar]
- Rengifo C, Rodríguez-Acosta A, 2005. Serpientes, Veneno y Tratamiento Médico en Venezuela. Fondo Editorial de la Facultad de Medicina Universidad Central de Venezuela, Caracas. [Google Scholar]
- Rinderknecht H, Geokas MC, Silverman P, Hanerback BJA, 1968. New ultrasensitive method for the determination of proteolytic activity. Clin. Chim. Acta 21, 197–203. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Acosta A, Sánchez EE, Márquez A, Carvajal Z, Salazar AM, Girón ME, Estrella A, Gil A, Guerrero B, 2010. Hemostatic properties of Venezuelan Bothrops snake venoms with special reference to Bothrops isabelae venom. Toxicon 56, 926–935. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Rodríguez-Acosta A, Girón ME, Aguilar I, Guerrero B, 2007. A comparative analysis of the clotting and fibrinolytic activities of the mapanare (Bothrops atrox) snake venom from different geographical areas in Venezuela. Thromb. Res 120, 95–104. [DOI] [PubMed] [Google Scholar]
- Sánchez EF, Schneider FS, Yarlequé A, Borges MH, Richardson M, Figueiredo SG, Evangelista KS, Eble JA, 2010. The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergón) snake venom acts both on blood vessel ECM and platelets. Arch. Biochem. Biophys 496, 9–20. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Girón ME, Uzcátegui NL, Guerrero B, Saucedo M, Cuevas E, Rodríguez-Acosta A, 2014. Biochemical and biological characterisation of lancehead (Bothrops venezuelensis Sandner 1952) snake venom from the Venezuelan Central Coastal range. Bol. Malariol. Sal. Ambient 54, 138–149. [PMC free article] [PubMed] [Google Scholar]
- Saviola AJ, Gandara AJ, Bryson RW Jr., Mackessy SP, 2017. Venom phenotypes of the rock rattlesnake (Crotalus lepidus) and the ridge-nosed rattlesnake (Crotalus willardi) from México and the United States. Toxicon 138, 119–129. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G, 1987. Tricineesodium dodecyl sulfatepolyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem 166, 368–379. [DOI] [PubMed] [Google Scholar]
- Serrano SM, Maroun RC, 2005. Snake venom serine proteinases: sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 45, 1115–1132. [DOI] [PubMed] [Google Scholar]
- Shannon JD, Baramova EN, Bjarnason JB, Fox JW, 1989. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J. Biol. Chem 264, 11575–11583. [PubMed] [Google Scholar]
- Spearman-Kärber R, 1978. Alternative methods of analysis for quantal responses. In: Finney D (Ed.), Statistical Method in Biological Assay Charles Griffin, London, U.K. [Google Scholar]
- Theakston RDG, Reid HA, 1983. Development of simple standard assay procedures for the characterization of snake venoms. Bull. World Health Organ 61, 949–956. [PMC free article] [PubMed] [Google Scholar]
- Tu AT, Gordon PJ, Chua A, 1965. Some biochemical evidence in support of the classification of the venomous snake. Toxicon 3, 5–8. [DOI] [PubMed] [Google Scholar]
- Woolfit M, Bromham L, Antunes TC, Yamashita KM, Barbaro KC, Saiki M, Santoro ML, 2010. Comparative analysis of newborn and adult Bothrops jararaca snake venoms. Toxicon 56, 1443–1458. [DOI] [PubMed] [Google Scholar]


