Figure 2.
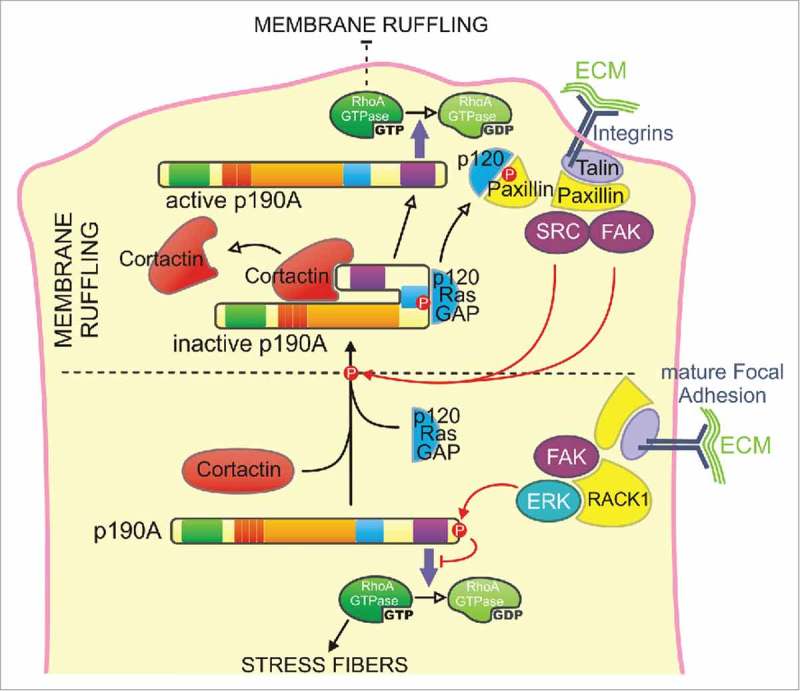
Model for the regulation of p190A targeting and activity at the cell leading edge - Two cooperative mechanisms may control p190A targeting to membrane protrusions. Cortactin binding to the PLS domain constitutively targets p190A in a closed/inactive conformation from the cytoplasm to the leading edge. Cortactin dissociation will result in the opening of the molecule, which locally turns off RhoA, thus allowing the maintenance of the newly expended membrane protrusion. Upon adhesion of the protrusion to the substratum, integrin signaling leads to the phosphorylation of p190A by Src or FAK on its p120BD and subsequent interaction with p120RasGAP (p120) that locally enhances p190A recruitment to the leading edge. p120RasGAP-paxillin interaction thus releases p190A that inactivates RhoA to limit premature adhesion maturation by stress fibers assembly. At the rear of the lamellipodia, recruitment of active ERK by RACK1 to mature focal adhesions induces C-terminal inhibitory phosphorylation of p190A, allowing RhoA to promote the formation of stress fibers.
