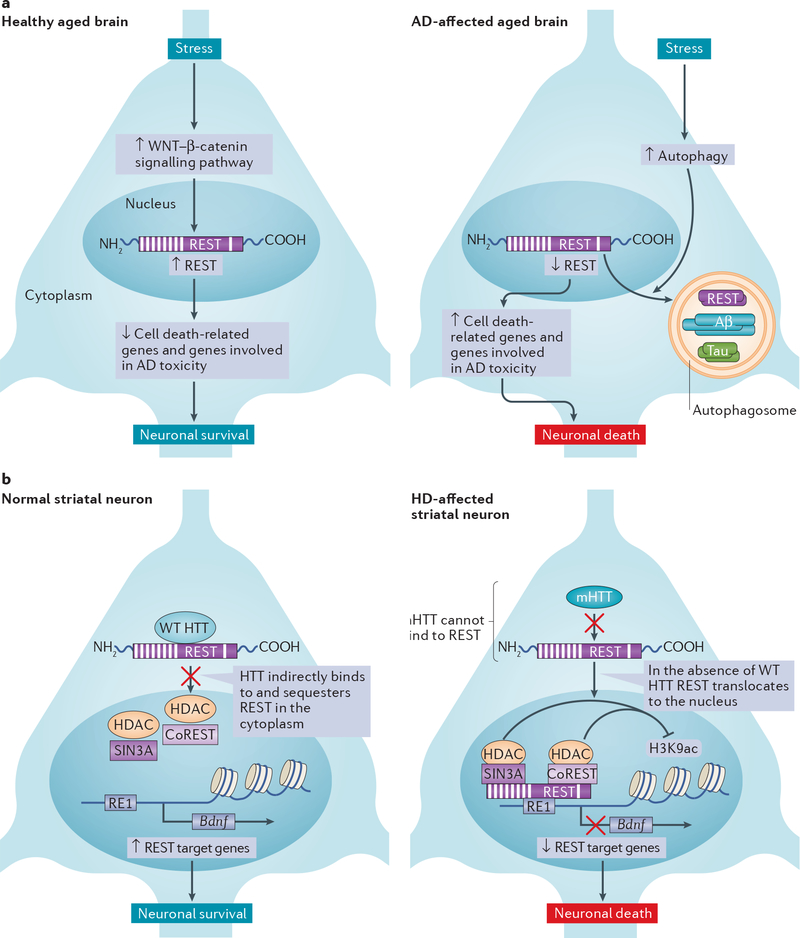
Figure 2 |. Restrictive element 1-silencing transcription factor in neurodegenerativo disease.
a | In -the healthy aged brain, stress increases WNT-β-catenin signalling (specifically that mediated by WNT3A and WNT7A) in the hippocampus and the frontal cortex. This promotes the nuclear translocation of restrictive element 1-silencing transcription factor (REST) and increased binding of REST to the restrictive element 1 (RE1) sites of REST target genes. This induction of REST nuclear abundance promotes neuroprotection by repressing the transcription of genes that encode proteins involved in neuronal death (such as BCL-2-binding component 3 (BBC3), BAX, death domain-associated protein 6 (DAXX), tumour necrosis factor receptor type 1-associated death domain protein (TRADD) and BCL-2-like protein 11 (BCL2L11)) and genes that encode proteins involved in Alzheimer disease (AD) pathology (such as members of the γ-secretase complex, which is implicated in amyloid-β (Aβ) generation). In AD-affected brains, oxidative stress activates autophagy129. The autophagosomes that are generated engulf REST, together with misfolded proteins such as Aβ and tau, which reduces REST abundance in the nucleus. The loss of REST in the nucleus causes an increase in expression of genes that are involved in oxidative stress and neuronal death. b | In the striatal neurons of wild-type (WT) mice, huntingtin (HTT) binds to and sequesters REST in the cytoplasm away from target genes that are important for neuronal activity and survival, such as brain-derived neurotrophic factor (BDNF)71,130,131. In the striatal neurons of mice carrying Huntington disease (HD)-related mutations, mutant HTT (mHTT) is unable to bind to and sequester REST, enabling REST to translocate to the nucleus where it silences target genes, thus promoting neuronal death41,42. A recent study suggests that this mechanism may not apply to neurons, but rather to glial cells, in the striata of humans with HD43. CoREST, REST co-repressor; H3K9ac, histone H3 lysine 9 acetylation; HDAC, histone deacetylase.