Figure 2.
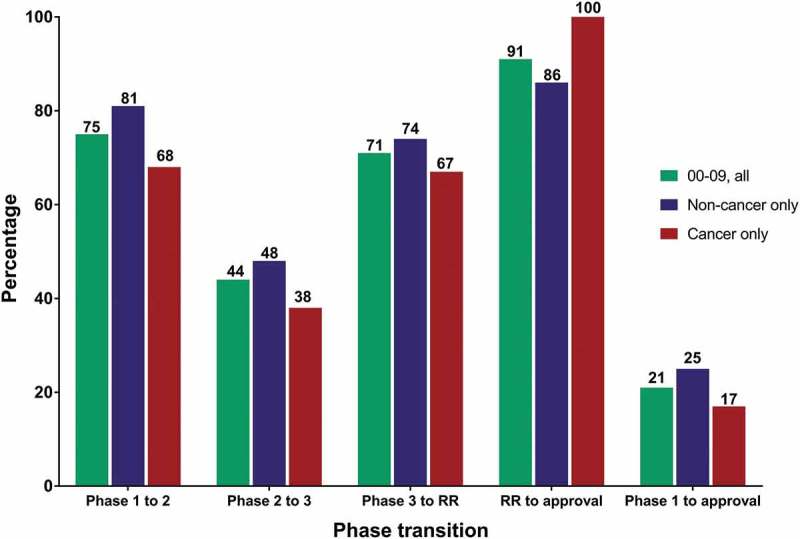
Clinical phase transition and approval success rates for antibody therapeutics that entered clinical study during 2000–2009.
Green bars, all antibody therapeutics. Blue bars, antibody therapeutics for non-cancer indications only. Red bars, antibody therapeutics for cancer only. Cohorts included only antibody therapeutics sponsored by commercial firms; those sponsored solely by government, academic or non-profit organizations were excluded. Number of molecules that entered clinical study during 2000–09: all, n = 357; non-cancer only, n = 181; cancer only, n = 176. Final fates (approval or termination) are known for 76%. MAbs that had advanced to Phase 1/2 were classified as Phase 2; mAbs that had advanced to Phase 2/3 were classified as Phase 3. Two mAbs with first approvals outside the US/EU regions (italizumab (Alzumab) and Rabishield approvals in India) were classified as Phase 3. Phase transition percentages were calculated as follows: the number of antibody therapeutics that completed a given phase and transitioned to the next was divided by the arithmetic difference between the number that entered the phase and the number that remained in the phase at the time of the calculation. Phase transitions occurring between clinical studies conducted world-wide were included. Approval success were defined as a first US or EU approval; supplemental approvals were not included. Abbreviation RR, regulatory review.
